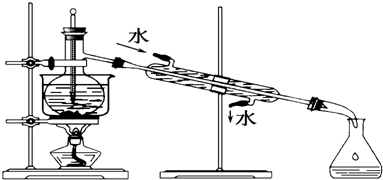
��Ŀ����
16���л�������A��C5H8O2��������ˮ�������Է�����ͼ��ʾ�ı仯��
��֪��C��һ�ȴ���Dֻ�����֣���ش�
��1��A�����к��еĹ����ŵ�����������̼̼˫��
��2���٢ڢܢݷ�Ӧ������ȡ����Ӧ���Тڢݣ�����ţ�
��3��C�Ľṹ��ʽCH3CH��CH3��COOH��X�Ľṹ��ʽ
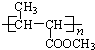
��4��C��ͬ���칹���������������4�֣�д���������ֵĽṹ��ʽCH3CH2COOCH3��CH3COOCH2CH3
��5��������֤D�к�����Ԫ�صķ���ȡ������D�����м�������������Һ��������У��ټ��������ữ��������������Һ���а�ɫ�������ɣ�֤��������Ԫ��
��6��17.2g B��������̼��������Һ��Ӧ����״�������ɶ�����̼���4.48 L��
���� ��������A��C5H8O2��������ˮ��Aת���õ��״���B��Ӧ�Ƿ�������ˮ�ⷴӦ��˵��A��������B��������������Һ��Ӧ����B�������ᣬA�����γɸ߾���X�����A�ķ���ʽ��˵��A������̼̼˫����Aˮ��������ƻ���ֻ����������̼̼˫��������������B��Ӧ������C=C˫����B�������������ӳɷ�ӦҲ˵����B����C=C˫��������A�ķ�������5��̼ԭ�ӣ�����ˮ���������˼״���B�����B����4��̼ԭ�ӣ�Bת����C�Ĺ�����û��̼ԭ�ӵı仯����C��Ҳֻ��4��̼ԭ�ӣ�C��һ�ȴ���D�����֣��ó�C�Ľṹ��ʽΪCH3CH��CH3��COOH����BΪCH2=C��CH3��COOH��AΪCH2=C��CH3��COOCH3��A�Ӿ۷�Ӧ���ɸ߾���X�Ľṹ��ʽ�ǣ�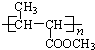 ���ݴ˽��
���ݴ˽��
��� �⣺��������A��C5H8O2��������ˮ��Aת���õ��״���B��Ӧ�Ƿ�������ˮ�ⷴӦ��˵��A��������B��������������Һ��Ӧ����B�������ᣬA�����γɸ߾���X�����A�ķ���ʽ��˵��A������̼̼˫����Aˮ��������ƻ���ֻ����������̼̼˫��������������B��Ӧ������C=C˫����B�������������ӳɷ�ӦҲ˵����B����C=C˫��������A�ķ�������5��̼ԭ�ӣ�����ˮ���������˼״���B�����B����4��̼ԭ�ӣ�Bת����C�Ĺ�����û��̼ԭ�ӵı仯����C��Ҳֻ��4��̼ԭ�ӣ�C��һ�ȴ���D�����֣��ó�C�Ľṹ��ʽΪCH3CH��CH3��COOH����BΪCH2=C��CH3��COOH��AΪCH2=C��CH3��COOCH3��A�Ӿ۷�Ӧ���ɸ߾���X�Ľṹ��ʽ�ǣ�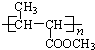 ��
��
��1��������������֪��AΪCH2=C��CH3��COOCH3��A�����к��еĹ����ţ�������̼̼˫�����ʴ�Ϊ��������̼̼˫����
��2����Ӧ�����ڼӾ۷�Ӧ����Ӧ�ڢ�����ȡ����Ӧ����Ӧ�����ڼӳɷ�Ӧ���ʴ�Ϊ���ڢݣ�
��3��������������֪��C�Ľṹ��ʽΪCH3CH��CH3��COOH��X�ṹ��ʽΪ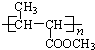 ���ʴ�Ϊ��CH3CH��CH3��COOH��
���ʴ�Ϊ��CH3CH��CH3��COOH��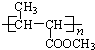 ��
��
��4��C��ͬ������������������У�CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH2CH2CH3��HCOOCH��CH3��2���ʴ�Ϊ��4��CH3CH2COOCH3��CH3COOCH2CH3��
��5������D�к�����Ԫ�صķ���Ϊ��ȡ������D�����м�������������Һ��������У��ټ��������ữ��������������Һ���а�ɫ�������ɣ�֤��������Ԫ�أ�
�ʴ�Ϊ��ȡ������D�����м�������������Һ��������У��ټ��������ữ��������������Һ���а�ɫ�������ɣ�֤��������Ԫ�أ�
��7��ΪCH2=C��CH3��COOH��17.2gB�����ʵ���Ϊ$\frac{17.2g}{86g/mol}$=0.2mol����������̼��������Һ��Ӧ�����ɶ�����̼Ϊ0.2mol����״�������ɶ�����̼�����Ϊ0.2mol��22.4L/mol=4.48L���ʴ�Ϊ��4.48��
���� ���⿼���л�����ƶϣ��漰����ϩ�������������ת�����Ѷ��еȣ��������Ʒ������Ʒ�����ƶϣ��ؼ��Ǹ��ݷ�Ӧ�ж�A���еĹ����ţ��ٽ��C��һ�ȴ��������֣��ó�C�Ľṹ��ʽ��

 һ����������ϵ�д�
һ����������ϵ�д�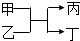 ���ס���Ϊ�����ڡ�ͬһ����Ԫ����ɵĵ��ʣ��ҡ�������������Ԫ����ɵĻ��������֮������ͼ��ʾ��ת����ϵ�������������ļͱ�����Ϊ��������
���ס���Ϊ�����ڡ�ͬһ����Ԫ����ɵĵ��ʣ��ҡ�������������Ԫ����ɵĻ��������֮������ͼ��ʾ��ת����ϵ�������������ļͱ�����Ϊ��������| A�� | �ƺ����� | B�� | ������� | C�� | ̼�� | D�� | �������嵥�� |
| A�� | 6.0gSiO2�������0.2NA��Si-O�� | |
| B�� | 1L1mol•L-1CH3COOH��Һ�У�����CH3COO-��CH3COOH������ΪNA | |
| C�� | 1L1mol•L-1����FeCl3��Һ�����ˮ����ȫˮ������Fe��OH��3����������ΪNA�� | |
| D�� | 10g46%���Ҵ�ˮ��Һ������Hԭ����Ϊ0.6NA |
| A�� | 2CaSO4•H2O��CaSO4•2H2O | B�� | CH3CH2OH��CH3OCH3 | ||
| C�� | CuSO4•3H2O��CuSO4•5H2O | D�� | H2O��D2O����ˮ�� |
| A�� | ��Ȼ�� �ƾ� Һ̬�Ȼ��� | B�� | ϡ������ Ũ���� ���� | ||
| C�� | ˮ�� �������� ���ùܵ�ú�� | D�� | ���ʯ ����ʯ ����ʯ |
| A�� | ������ζʱҪС�Ľ�����ƿ���ڱǿ���ֱ���� | |
| B�� | ��������Ư���ԣ�������ʹ�ʻ���ɫ | |
| C�� | ��ͨ������£��������Ժ�����ͭ�Ƚ���ֱ�ӻ��� | |
| D�� | ����й©ʱ����պ��̼������Һ��ë�����ڱǿ���Χ |