��Ŀ����
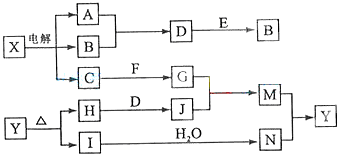
��12����֪A��IΪ��ѧ��ѧ�������ʣ�ͨ������£���CΪ�����⣬�������ʾ�Ϊ���塣��֪A��BΪ�������ʣ���ҵ����ͨ��ֽ�������H��ұ������A��F�Ǻ���ɫ��ĩ���������ת����ϵ����ͼ��ʾ��
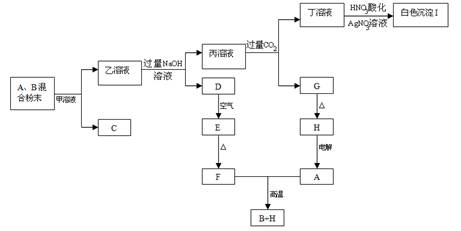
�ش��������⣺
��1������Һ���ʵĻ�ѧʽ��___________________��
��2��д��������Һ�м������NaOH��Һ��������Ӧ�����ӷ���ʽ��
��____________________________________________________________________��
��____________________________________________________________________��
��3��д��D��E��ʵ������ͻ�ѧ��Ӧ����ʽ:
_______________________________________________________________________
_______________________________________________________________��
��4�� ���F��Aǡ����ȫ��Ӧ����B��H�����Ϸ�ĩ��A��B��������Ϊ__________ ��
��1��NaCl ��NaHCO3
��2���� Al3++4 OH![]() == AlO2
== AlO2![]() + 2 H2O
+ 2 H2O
�� Fe2++2 OH![]() == Fe��OH��2�� ���١��ڷ���ʽ��˳����Եߵ���
== Fe��OH��2�� ���١��ڷ���ʽ��˳����Եߵ���
��3�������ɰ�ɫ��Ϊ����ɫ������Ϊ���ɫ��
4Fe��OH��2 + O2 + 2 H2O == 4 Fe��OH��3
��4��27��56

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�