��Ŀ����
�����أ������ĺ��������Ϊ��ɫ��״���壬�����ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156��157�棬���ȶ��Բ�������Ǹ�Ч�Ŀ�űҩ����֪�����ѷе�Ϊ35�档����������ȡ�����صķ���֮һ������ȡԭ��Ϊ�����ģ���Ҫ�����ѽ�ȡ�������ͽ�ȡ�������ѽ�ȡ������Ҫ����Ϊ��
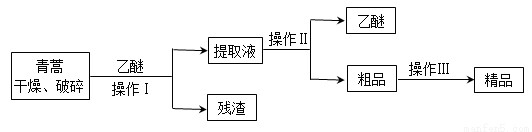
��ش��������⣺
�Ŷ�������и��������Ŀ���� ��
�Ʋ���I��Ҫ�IJ���������Ҫ�У��ձ���©���� ��������������� ��
�Dz��������Ҫ���̿�����_____________������ĸ����
A����ˮ�ܽ⣬����Ũ������ȴ�ᾧ
B����95�����Ҵ���Ũ�����ᾧ������
C���������ѽ�����ȡ��Һ
��������ʵ��װ�òⶨ�����ط���ʽ�ķ������£�
��28.2g��������Ʒ����Ӳ�ʲ�����C�У�����ͨ����������Ӻ��ٳ��ȼ�գ���ȷ�ⶨװ��E��Fʵ��ǰ��������������������ݼ��㡣
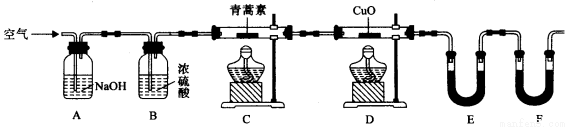
��װ��E��ʢ�ŵ������� ��
�ڸ�ʵ��װ�ÿ��ܻ��������ɲⶨ������ƫ�ͣ��Ľ������� ��
���ú����Ľ����װ�ý������飬�Ƶã�
װ�� | ʵ��ǰ/g | ʵ���/g |
E | 22.6 | 42.4 |
F | 80.2 | 146.2 |
���������ص����ʽ�� ��
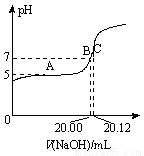
��ijѧ���������ص����ʽ���̽�����������ؼ��뺬��NaOH����̪��ˮ��Һ�У������ص��ܽ����� С�����Ȳ����裬�����ص��ܽ�����������Һ��ɫ��dz��˵���������� ������ĸ��������ͬ�����ʡ�
С�����Ȳ����裬�����ص��ܽ�����������Һ��ɫ��dz��˵���������� ������ĸ��������ͬ�����ʡ�
A���Ҵ� B������ C���������� D��������
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�

 ��������)��
��������)��
 Na2S(s)+4H2O(g)��
Na2S(s)+4H2O(g)�� SO2��C2H4��O2������
SO2��C2H4��O2������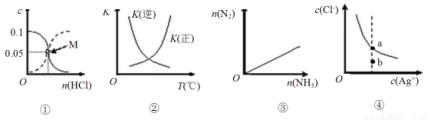
 2SO3(g) ��H��0���淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
2SO3(g) ��H��0���淴Ӧ��ƽ�ⳣ��K���¶ȵı仯 aq)��Cl��(aq)�����ӵ�Ũ�ȹ�ϵ��������b��ʱ���������ֵ�ˮ���Ե���ƽ���ߵ�a�㴦
aq)��Cl��(aq)�����ӵ�Ũ�ȹ�ϵ��������b��ʱ���������ֵ�ˮ���Ե���ƽ���ߵ�a�㴦