��Ŀ����
��ˮ�������Ƹ����������ȵ�600��㿪ʼ�ֽ⣬�ֽ���������ƺ�����һ�ֹ��塣ijѧ������ˮ�����������Ⱥ����������ˮ�Ƴ�Ũ��Һ������ͼ��ʾ��ʵ��װ�ý���ʵ�飬����������Һ�л�������μ���ϡ���ᣬ����ʵ�������жϹ�����������ʲô�ɷ֣�
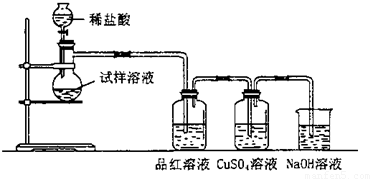
��1����������¶���600�����ϣ��������ù����������Һ�л����μ�ϡ�������������۲쵽Ʒ����Һ����ɫ��CuSO4��Һ�г��ֺ�ɫ�������Է���������Һ�п��ܳ��ֵ�����__________ _________������������������ӷ���ʽ��_____________ _ _��Ʒ����Һ����ɫ��ԭ���ǣ�_____________________��
��2��ͨ������ʵ�������ƶ���ˮ�����������ȷֽ�Ļ�ѧ����ʽ��____________________��װ���е�NaOH��Һ��������____________________________��
��3����������������ϡ�����Ʒ����Һ��CuSO4��Һ�ж������Ե���������ԭ����_____________________________________��
��1���е���ɫ�������ɣ���������ð��

SO32- + 2S2- + 6H+ == 3S�� + 3H2O S2- + 2H+ == H2S��
���Ⱥ������������Na2SO3��Na2S�����ʵ���֮��С��1:2����������Һ�з�Ӧ����������ֻ��H2S��������SO2��
��2��4Na2SO3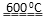 Na2S + 3Na2SO4;
����H2S��SO2����
Na2S + 3Na2SO4;
����H2S��SO2����
��3�����Ⱥ����������Na2SO3��Na2S�����ʵ���֮�ȵ���1:2����������Һ��ǡ������S
����������1��CuSO4��Һ�г��ֺ�ɫ������˵�������������塣Ʒ����Һ����ɫ��˵��û������SO2���������ڼ��Ⱥ������������Na2SO3��Na2S�����ʵ���֮��С��1:2����������Һ�з�Ӧ����������ֻ��H2S��������SO2����ӦʽΪSO32- + 2S2- + 6H+ == 3S�� + 3H2O ��S2- + 2H+ == H2S����
��2�����������ɣ�˵���ֽⷴӦ��������ԭ��Ӧ����������һ�ֹ����������ƣ���ӦʽΪ4Na2SO3 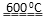 Na2S + 3Na2SO4�������������SO2�������ڴ�����Ⱦ���Ҫβ������������������������������H2S��SO2����ġ�
Na2S + 3Na2SO4�������������SO2�������ڴ�����Ⱦ���Ҫβ������������������������������H2S��SO2����ġ�
��3�����ݷ�ӦSO32- + 2S2- + 6H+ == 3S�� + 3H2O��֪��������Ⱥ����������Na2SO3��Na2S�����ʵ���֮�ȵ���1:2������������Һ��ǡ������S������Ʒ����Һ��CuSO4��Һ�ж������Ե�������

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�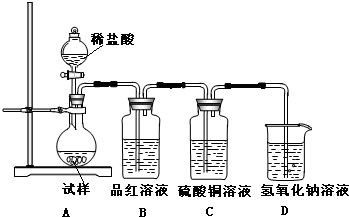 Ϊ̽���������Ƶ����ȶ��ԣ�ij�о���ѧϰС�齫��ˮ�������Ƹ����������ȣ����������Ⱥ�Ĺ���������ͼ��ʾ��ʵ��װ�ý���ʵ�飮��ش������й����⣺
Ϊ̽���������Ƶ����ȶ��ԣ�ij�о���ѧϰС�齫��ˮ�������Ƹ����������ȣ����������Ⱥ�Ĺ���������ͼ��ʾ��ʵ��װ�ý���ʵ�飮��ش������й����⣺ ��ˮ�������Ƹ����������ȵ�600��㿪ʼ�ֽ⣬�ֽ���������ƺ�����һ�ֹ��壮ijѧ������ˮ�����������Ⱥ����������ˮ�Ƴ�Ũ��Һ������ͼ��ʾ��ʵ��װ�ý���ʵ�飬����������Һ�л�������μ���ϡ���ᣬ����ʵ�������жϹ�����������ʲô�ɷ֣�����֪��Na2 S+2HCl�T2NaCl+H2S+SO2+2H2S�T3S+2H2 O��
��ˮ�������Ƹ����������ȵ�600��㿪ʼ�ֽ⣬�ֽ���������ƺ�����һ�ֹ��壮ijѧ������ˮ�����������Ⱥ����������ˮ�Ƴ�Ũ��Һ������ͼ��ʾ��ʵ��װ�ý���ʵ�飬����������Һ�л�������μ���ϡ���ᣬ����ʵ�������жϹ�����������ʲô�ɷ֣�����֪��Na2 S+2HCl�T2NaCl+H2S+SO2+2H2S�T3S+2H2 O��
