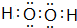��Ŀ����
 �±���Ԫ�����ڱ���ǰ�����ڣ�
�±���Ԫ�����ڱ���ǰ�����ڣ�| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| һ | A | |||||||
| �� | B | C | D | E | F | |||
| �� | G | H | I | J |
��1��д������Ԫ�ص�Ԫ�صķ��ţ�F
F
F
��JAr
Ar
����2��GԪ����EԪ���γɵĻ�����Ļ�ѧʽ��
Na2O
Na2O
��Na2O2
Na2O2
�����Ƕ�������
����
������ӡ����ۡ����������3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����
HClO4
HClO4
���������������������Al��OH��3
Al��OH��3
���û�����Ļ�ѧʽ��ʾ������4��ֻ����A��C����Ԫ�صĻ������Ϊ
��
��
����Щ�������У�����Է���������С����
CH4
CH4
���û�����ķ��ӿռ乹������������
��������
���ڷ����к���˫������ԭ�������ٵ���
CH2=CH2
CH2=CH2
������HCl��Ӧ�Ļ�ѧ����ʽΪCH2=CH2+HCl��CH3CH2Cl
CH2=CH2+HCl��CH3CH2Cl
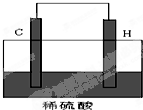
����5����H������C��һ�ֵ��ʣ����壩����ͼװ�����ӣ���װ�ó�Ϊ
ԭ���
ԭ���
������C������
����
����������������д���õ缫��ӦʽΪ��2H++2e-=H2��
2H++2e-=H2��
��������������H����C
H����C
����������Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪLi��CΪC��DΪN��EΪO��FΪF��GΪNa��HΪAl��IΪCl��JΪAr��Ȼ����Ԫ�ؼ��䵥�ʡ�����������ʼ���ѧ���������
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪLi��CΪC��DΪN��EΪO��FΪF��GΪNa��HΪAl��IΪCl��JΪAr��
��1��������������֪��FΪFԪ�أ�JΪArԪ�أ��ʴ�Ϊ��F��Ar��
��2��GԪ����EԪ���γɵĻ�����Ļ�ѧʽΪNa2O��Na2O2���������Ӽ����������ӻ�����ʴ�Ϊ��Na2O��Na2O2�����ӣ�
��3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����HClO4���������������������Al��OH��3���ʴ�Ϊ����Al��OH��3��
��4��A��C����Ԫ�صĻ������Ϊ�����ʴ�Ϊ������
����Է���������С����CH4����ռ乹��Ϊ�������壬�ʴ�Ϊ��CH4���������壻
�ڷ����к���˫������ԭ�������ٵ���CH2=CH2����HCl�����ļӳɷ�ӦΪCH2=CH2+HCl��CH3CH2Cl���ʴ�Ϊ��CH2=CH2��CH2=CH2+HCl��CH3CH2Cl��
��5����ͼ��֪������ΪAl������ΪC�������Ϊ���ᣬװ�ù���ԭ��أ�������ӦΪ2H++2e-=H2���������ɸ���������������ͼ����H����C��
�ʴ�Ϊ��ԭ��أ�������2H++2e-=H2����H����C��
��1��������������֪��FΪFԪ�أ�JΪArԪ�أ��ʴ�Ϊ��F��Ar��
��2��GԪ����EԪ���γɵĻ�����Ļ�ѧʽΪNa2O��Na2O2���������Ӽ����������ӻ�����ʴ�Ϊ��Na2O��Na2O2�����ӣ�
��3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����HClO4���������������������Al��OH��3���ʴ�Ϊ����Al��OH��3��
��4��A��C����Ԫ�صĻ������Ϊ�����ʴ�Ϊ������
����Է���������С����CH4����ռ乹��Ϊ�������壬�ʴ�Ϊ��CH4���������壻
�ڷ����к���˫������ԭ�������ٵ���CH2=CH2����HCl�����ļӳɷ�ӦΪCH2=CH2+HCl��CH3CH2Cl���ʴ�Ϊ��CH2=CH2��CH2=CH2+HCl��CH3CH2Cl��
��5����ͼ��֪������ΪAl������ΪC�������Ϊ���ᣬװ�ù���ԭ��أ�������ӦΪ2H++2e-=H2���������ɸ���������������ͼ����H����C��
�ʴ�Ϊ��ԭ��أ�������2H++2e-=H2����H����C��
���������⿼��λ�á��ṹ�����ʵȣ��漰Ԫ�����ڱ���Ԫ�������ɣ���ȷԪ�ؼ����ʡ���������·��Ӽ��ɽ��ע�ػ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ


 �±���Ԫ�����ڱ���ǰ�����ڣ�
�±���Ԫ�����ڱ���ǰ�����ڣ�