��Ŀ����
��10�֣���������͵����������Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��
�� һ�������£�2SO2(g)��O��(g)![]() 2SO3(g)����2L�ܱ�������ͨ��2mol SO2(g)��1mol O2(g)��0.2mol SO3(g)��2min��Ӧ�ﵽƽ��ʱ�����SO2��ת����Ϊ50������ÿ��淴Ӧ��ƽ�ⳣ��K�������������������������£������������ټ���2mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� 50����ѡ���������������������
2SO3(g)����2L�ܱ�������ͨ��2mol SO2(g)��1mol O2(g)��0.2mol SO3(g)��2min��Ӧ�ﵽƽ��ʱ�����SO2��ת����Ϊ50������ÿ��淴Ӧ��ƽ�ⳣ��K�������������������������£������������ټ���2mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� 50����ѡ���������������������
![]() �� ��CH4����ԭNOxΪN2�������������������Ⱦ����д���ܷ�Ӧ����ʽ�� ������1L NO2��NO�������NOx���仹ԭ��N2����ͬ��ͬѹ��CH4�����0.
�� ��CH4����ԭNOxΪN2�������������������Ⱦ����д���ܷ�Ӧ����ʽ�� ������1L NO2��NO�������NOx���仹ԭ��N2����ͬ��ͬѹ��CH4�����0.
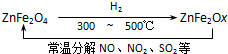
![]() �� �������ײ�����ȱλ�����Σ�ZnFe2Ox�����������Σ�ZnFe2O4�������»�ԭ�Ƶã������£�����ʹ��ҵ�����е�����������ֽ��ȥ��ת��������ͼ��ʾ��
�� �������ײ�����ȱλ�����Σ�ZnFe2Ox�����������Σ�ZnFe2O4�������»�ԭ�Ƶã������£�����ʹ��ҵ�����е�����������ֽ��ȥ��ת��������ͼ��ʾ��
![]()

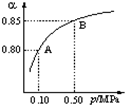
![]() ��2mol ZnFe2Ox��SO2������0.75mol S��x�� ������ ����ɷֽ���л������V�� ���������� L��
��2mol ZnFe2Ox��SO2������0.75mol S��x�� ������ ����ɷֽ���л������V�� ���������� L��
5.76 < xCH4+4NOx=2N2+xCO2+2xH2O 3:2=1.5 3.25 21

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�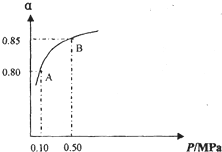 ��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��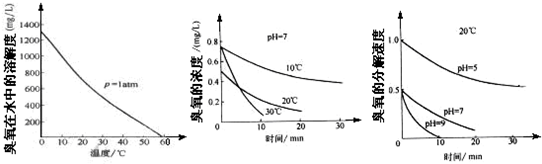


 ��2009?��˳ģ�⣩��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��2009?��˳ģ�⣩��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
 ��������͵����������Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��
��������͵����������Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��