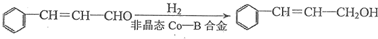��Ŀ����
�л��ϳ�·�����: �����������������ѡ��һ������
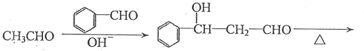
������֪��һ�������£������к��Ц�-H��ȩ�ᷢ�����Ӽ�ӳɷ�Ӧ��
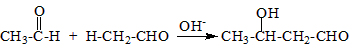
�����Լ��ȼ�ʧȥһ����ˮ��ɦ�-�²�����ȩ
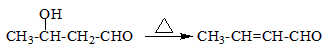
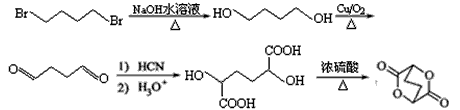
��ϸ�������Ϣ��ƺ���������CH3CH2Br�ϳ�CH3CH2CH2CH2OH�����÷�Ӧ����ͼ��ʾ�����ڼ�ͷ��ע����Ӧ���������Լ���ѡ����
������֪��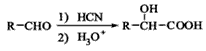 ��д����
��д���� �Ʊ�������
�Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ����
�ĺϳ�·������ͼ�����Լ���ѡ����
������֪��һ�������£������к��Ц�-H��ȩ�ᷢ�����Ӽ�ӳɷ�Ӧ��
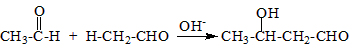
�����Լ��ȼ�ʧȥһ����ˮ��ɦ�-�²�����ȩ
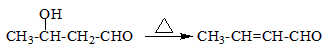
��ϸ�������Ϣ��ƺ���������CH3CH2Br�ϳ�CH3CH2CH2CH2OH�����÷�Ӧ����ͼ��ʾ�����ڼ�ͷ��ע����Ӧ���������Լ���ѡ����
������֪��
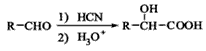 �����
��д���� �Ʊ�������
�Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ����
�ĺϳ�·������ͼ�����Լ���ѡ���� ����CH3CH2Br 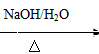 CH3CH2OH
CH3CH2OH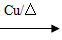 CH3CHO
CH3CHO  CH3CHOHCH2CHO
CH3CHOHCH2CHO 
CH3CH=CHCHO CH3CH2CH2CH2OH
CH3CH2CH2CH2OH
����
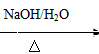 CH3CH2OH
CH3CH2OH CH3CHO
CH3CHO  CH3CHOHCH2CHO
CH3CHOHCH2CHO 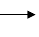
CH3CH=CHCHO
 CH3CH2CH2CH2OH
CH3CH2CH2CH2OH ����


��ϰ��ϵ�д�
�����Ŀ
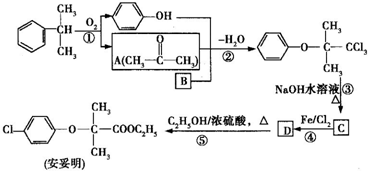
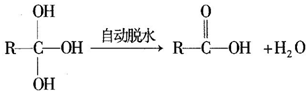
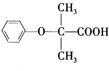
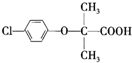 +CH3CH2OH
+CH3CH2OH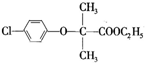 +H2O
+H2O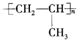 ���úϳ�·������ͼ��ʾ����ע����Ӧ��������
���úϳ�·������ͼ��ʾ����ע����Ӧ��������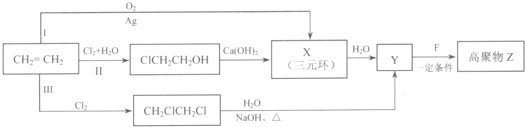
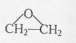
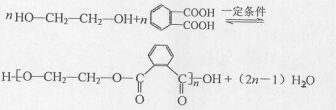
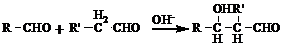 ��R��R'������������ԭ�ӣ�
��R��R'������������ԭ�ӣ�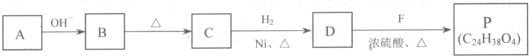
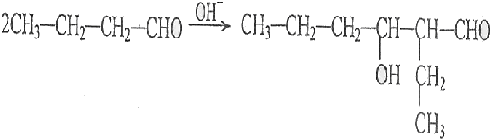
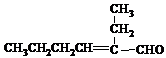
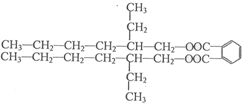
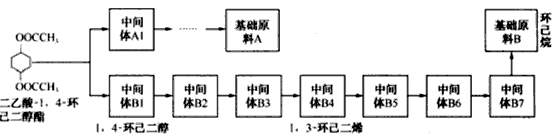
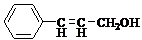 ���ĺϳ�·�ߣ�
���ĺϳ�·�ߣ�