��Ŀ����
[ѡ����]��1�����Ƕ�������ʶ�����м��������ʷ��������һ����dz����ɵͼ���������ʶ���̣�Ŀǰ��ѧ�α��е����������1889�갢������˹��Arrhenius������ĵ������ۣ�
��1905�긻�����֣�FranKlin�������о���ˮ��Һ���������ԣ��Ѱ�������˹��ˮΪ�ܼ��ĸ��������ƹ㵽�κ��ܼ������������ܼ����ۣ��ܼ�������Ϊ�����ܵ���������ܼ������ӵ�����Ϊ�ᣬ���ܵ���������ܼ������ӵ�����Ϊ���д��Һ����������ķ���ʽ��
��1923�굤��ѧ�Ҳ���˹�أ�Bronsted����Ӣ����ѧ��������Lowey������������ۣ������ܹ��ͷ����ӣ������ӣ��κκ���ԭ�ӵķ��ӻ����Ӷ���������ܹ������ӣ������ӣ���ϵķ��ӻ����Ӷ��Ǽ
���������ۣ�����������ˮ��Һ�ȿ��Կ������ֿɿ������
A��H2O B��NH4+ C��OH- D��HCO3- E��CH3COO- F��Cl-
��1923��·��˹��Lewis��������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����ᣮ
�ᣨ���ӶԽ����壩+����ӶԸ����壩����Ӧ����磺H++OH-��H2O��
��ָ������������Ӧ�е����
����H3BO3+H2O H++B��OH��4-���÷�Ӧ�еļ���
H++B��OH��4-���÷�Ӧ�еļ���
����NaH+H2O�TNaOH+H2�����÷�Ӧ�е�����
��2����֪AԪ��ԭ�ӵ�K��L�������֮�ͱ�M��L�������֮�Ͷ�һ�����ӣ�BԪ��ԭ�Ӻ������ռ��9�����������1��δ�ɶԵ��ӣ�CԪ��ԭ�Ӻ���3p�Dz���3���������5�����Ӳ��ܴﵽȫ������DԪ��ֻ���������Ӳ㣬������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣻Eԭ�Ӱ뾶��С��FԪ�����������Ų�ΪnSnnPn+1����Ҫ����д
��B�ĵ����Ų�ʽ�ǣ�
��DԪ�ص��ʵľ���������
��E��F�γɵĻ�����ռ乹��Ϊ
��1905�긻�����֣�FranKlin�������о���ˮ��Һ���������ԣ��Ѱ�������˹��ˮΪ�ܼ��ĸ��������ƹ㵽�κ��ܼ������������ܼ����ۣ��ܼ�������Ϊ�����ܵ���������ܼ������ӵ�����Ϊ�ᣬ���ܵ���������ܼ������ӵ�����Ϊ���д��Һ����������ķ���ʽ��
2NH3=NH4++NH2-
2NH3=NH4++NH2-
����1923�굤��ѧ�Ҳ���˹�أ�Bronsted����Ӣ����ѧ��������Lowey������������ۣ������ܹ��ͷ����ӣ������ӣ��κκ���ԭ�ӵķ��ӻ����Ӷ���������ܹ������ӣ������ӣ���ϵķ��ӻ����Ӷ��Ǽ
���������ۣ�����������ˮ��Һ�ȿ��Կ������ֿɿ������
AD
AD
��A��H2O B��NH4+ C��OH- D��HCO3- E��CH3COO- F��Cl-
��1923��·��˹��Lewis��������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����ᣮ
�ᣨ���ӶԽ����壩+����ӶԸ����壩����Ӧ����磺H++OH-��H2O��
��ָ������������Ӧ�е����
����H3BO3+H2O
 H++B��OH��4-���÷�Ӧ�еļ���
H++B��OH��4-���÷�Ӧ�еļ���H2O
H2O
����H3BO3��H2O��������NaH+H2O�TNaOH+H2�����÷�Ӧ�е�����
H2O
H2O
����NaH ��H2O������2����֪AԪ��ԭ�ӵ�K��L�������֮�ͱ�M��L�������֮�Ͷ�һ�����ӣ�BԪ��ԭ�Ӻ������ռ��9�����������1��δ�ɶԵ��ӣ�CԪ��ԭ�Ӻ���3p�Dz���3���������5�����Ӳ��ܴﵽȫ������DԪ��ֻ���������Ӳ㣬������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣻Eԭ�Ӱ뾶��С��FԪ�����������Ų�ΪnSnnPn+1����Ҫ����д
��B�ĵ����Ų�ʽ�ǣ�
1s22s22p63s23p5
1s22s22p63s23p5
��A��B��Ԫ���γɵĻ�����ľ������������Ӿ���
���Ӿ���
����DԪ�ص��ʵľ���������
ԭ�Ӿ���
ԭ�Ӿ���
��C���ʵ��Ʊ������ǣ���ⷨ
��ⷨ
����E��F�γɵĻ�����ռ乹��Ϊ
������
������
�������ʱ�D��E�γɵĻ����������Һ����ԭ����E��F�γɵĻ�������Ӽ����γ����
E��F�γɵĻ�������Ӽ����γ����
����������1��������֪ʶǨ�ƣ�ˮ�ĵ��룩������������
�ڸ����������۷���������������ӵ����ᣬ��������ӵ��Ǽ
�۸��ݹ�������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����
��2����������֪��AԪ����3�����Ӳ㣬�������7�����ӣ��ɴ��ж�AԪ�����ƣ����ݵ����Ų�ʽ�����ж�BԪ�أ������������ԭ����������ж�CԪ�أ���������Ԫ�ص�������=����������������������ϼ�+������ϼ۵ľ���ֵ=8������������ж�D��Eԭ�Ӱ뾶��С��������HԪ�أ�����S�ܼ�������ĵ������ж�n���Ӷ��жϸ�Ԫ�أ�Ȼ��������������⣮
�ڸ����������۷���������������ӵ����ᣬ��������ӵ��Ǽ
�۸��ݹ�������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����
��2����������֪��AԪ����3�����Ӳ㣬�������7�����ӣ��ɴ��ж�AԪ�����ƣ����ݵ����Ų�ʽ�����ж�BԪ�أ������������ԭ����������ж�CԪ�أ���������Ԫ�ص�������=����������������������ϼ�+������ϼ۵ľ���ֵ=8������������ж�D��Eԭ�Ӱ뾶��С��������HԪ�أ�����S�ܼ�������ĵ������ж�n���Ӷ��жϸ�Ԫ�أ�Ȼ��������������⣮
����⣺��1����ˮ���ӵ����ˮ�������Ӻ����������ӣ�������ȷ���Һ����������ķ���ʽ2NH3=NH4++NH2-��
�ʴ�Ϊ��2NH3=NH4++NH2-��
�ڸ����������۷���������������ӵ����ᣬ��������ӵ��Ǽ
A��H2O D��HCO3- ���ܵ���������ӣ����ܽ�������ӣ����Լȿ��Կ������ֿɿ����
B��NH4+ �ܵ���������ӣ��������
C��OH-��E��CH3COO-��F��Cl- �ܽ�������ӣ������Ǽ
�ʴ�Ϊ��A D��
�۸��ݹ�������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����
����H3BO3+H2O H++B��OH��4-���÷�Ӧ�и������ӶԶ��γɻ�ѧ��������H2O�����Ը÷�Ӧ�еļ���H2O��
H++B��OH��4-���÷�Ӧ�и������ӶԶ��γɻ�ѧ��������H2O�����Ը÷�Ӧ�еļ���H2O��
�ʴ�Ϊ��H2O��
����NaH+H2O�TNaOH+H2�����÷�Ӧ�к͵��ӶԽ�ϵ�����H2O������ˮ���ᣮ
�ʴ�Ϊ��H2O��
��2����������֪��AԪ����3�����Ӳ㣬�������7�����ӣ�����A��Na��BԪ��ԭ�Ӻ������ռ��9�����������1��δ�ɶԵ��ӣ��ɴ��ж�B��Cl��CԪ��ԭ�Ӻ���3p�Dz���3���������5�����Ӳ��ܴﵽȫ����������CԪ�ص�3P�Dz���1�����ӣ�����C��Al��DԪ��ֻ���������Ӳ㣬������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣬����Ԫ�ص�������=����������������������ϼ�+������ϼ۵ľ���ֵ=8������D��C��Eԭ�Ӱ뾶��С������E��H��FԪ�����������Ų�ΪnSnnPn+1��S�ܼ������2�����ӣ���n��2������FԪ�����������Ų�Ϊ2S22P3��������N��
��ͨ�����Ϸ���֪��B��Cl���������������Ų�ʽΪ1s22s22p63s23p5��A��Na��B��Cl�����ý����ͻ��÷ǽ���Ԫ��֮���γ����Ӽ������������Ӿ��壻
�ʴ�Ϊ��1s22s22p63s23p5�� ���Ӿ��壮
��D��CԪ�أ�̼���ʵľ���������ԭ�Ӿ��壻C������Al�����ý������õ�ⷨұ����
�ʴ�Ϊ��ԭ�Ӿ��壻 ��ⷨ��
��E��HԪ�أ�F��NԪ�أ������γɵĻ�������NH3��NH3�Ŀռ乹��Ϊ�����ͣ�NH3���Ӽ����γ��������������Һ����
�ʴ�Ϊ�������ͣ� E��F�γɵĻ�������Ӽ����γ������
�ʴ�Ϊ��2NH3=NH4++NH2-��
�ڸ����������۷���������������ӵ����ᣬ��������ӵ��Ǽ
A��H2O D��HCO3- ���ܵ���������ӣ����ܽ�������ӣ����Լȿ��Կ������ֿɿ����
B��NH4+ �ܵ���������ӣ��������
C��OH-��E��CH3COO-��F��Cl- �ܽ�������ӣ������Ǽ
�ʴ�Ϊ��A D��
�۸��ݹ�������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����
����H3BO3+H2O
 H++B��OH��4-���÷�Ӧ�и������ӶԶ��γɻ�ѧ��������H2O�����Ը÷�Ӧ�еļ���H2O��
H++B��OH��4-���÷�Ӧ�и������ӶԶ��γɻ�ѧ��������H2O�����Ը÷�Ӧ�еļ���H2O���ʴ�Ϊ��H2O��
����NaH+H2O�TNaOH+H2�����÷�Ӧ�к͵��ӶԽ�ϵ�����H2O������ˮ���ᣮ
�ʴ�Ϊ��H2O��
��2����������֪��AԪ����3�����Ӳ㣬�������7�����ӣ�����A��Na��BԪ��ԭ�Ӻ������ռ��9�����������1��δ�ɶԵ��ӣ��ɴ��ж�B��Cl��CԪ��ԭ�Ӻ���3p�Dz���3���������5�����Ӳ��ܴﵽȫ����������CԪ�ص�3P�Dz���1�����ӣ�����C��Al��DԪ��ֻ���������Ӳ㣬������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣬����Ԫ�ص�������=����������������������ϼ�+������ϼ۵ľ���ֵ=8������D��C��Eԭ�Ӱ뾶��С������E��H��FԪ�����������Ų�ΪnSnnPn+1��S�ܼ������2�����ӣ���n��2������FԪ�����������Ų�Ϊ2S22P3��������N��
��ͨ�����Ϸ���֪��B��Cl���������������Ų�ʽΪ1s22s22p63s23p5��A��Na��B��Cl�����ý����ͻ��÷ǽ���Ԫ��֮���γ����Ӽ������������Ӿ��壻
�ʴ�Ϊ��1s22s22p63s23p5�� ���Ӿ��壮
��D��CԪ�أ�̼���ʵľ���������ԭ�Ӿ��壻C������Al�����ý������õ�ⷨұ����
�ʴ�Ϊ��ԭ�Ӿ��壻 ��ⷨ��
��E��HԪ�أ�F��NԪ�أ������γɵĻ�������NH3��NH3�Ŀռ乹��Ϊ�����ͣ�NH3���Ӽ����γ��������������Һ����
�ʴ�Ϊ�������ͣ� E��F�γɵĻ�������Ӽ����γ������
���������⿼�������ĸ�������Ų�ʽ�����ӵĿռ乹�͵�֪ʶ�㣬�ѶȲ���ӽ̲����ҳ�����֪ʶ��Ȼ������֪ʶǨ�Ƶķ���������⣮

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ
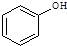 ��
��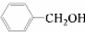 ��Ӧѡ��
��Ӧѡ��
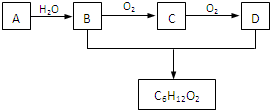

 ��2008?��ɽһģ��ѡ���⣺����ѧר��ͨ��ʵ�鷢�֣��ڴ��Ե���Ӧ��λ-���������ġ���������͵ĵ������ᴦ���ƺ����ȿ��ֵ�״̬�������Ѿ������������ġ����ֵ��Ե�ͼ���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ��������ġ��ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹ��ͼ����ش��������⣺
��2008?��ɽһģ��ѡ���⣺����ѧר��ͨ��ʵ�鷢�֣��ڴ��Ե���Ӧ��λ-���������ġ���������͵ĵ������ᴦ���ƺ����ȿ��ֵ�״̬�������Ѿ������������ġ����ֵ��Ե�ͼ���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ��������ġ��ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹ��ͼ����ش��������⣺




