��Ŀ����
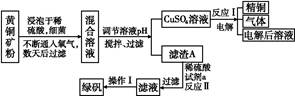
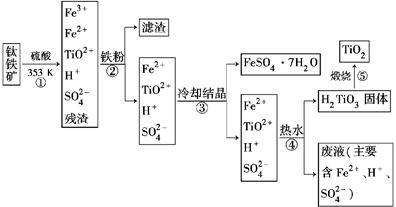
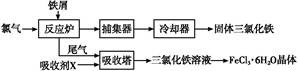
��ҵ����������Ϊԭ���Ʊ��������ѵĹ�����������ͼ��ʾ�����������Ҫ�ɷ�Ϊ��������(FeTiO3)������һ������Ԫ���ڷ绯�����л�ת��Ϊ��3�ۡ�
��֪��TiOSO4��ˮ��ˮ�⡣
(1)������У������۽�Fe3��ת��ΪFe2�������ӷ���ʽΪ_______________________
(2)������У�ʵ�ֻ����ķ������������ʵ�________(����ĸ���)��
a���ۡ��е���졡 b���ܽ��Բ��졡 c�������ԡ���ԭ�Բ���
(3)����ڡ��ۡ����У�����Ҫ���еIJ�����________(���������)��
(4)���ϻ�ѧ�����û�ѧƽ�����۽��Ͳ�����н�TiO2��ת��ΪH2TiO3��ԭ����
____________________________________________________________��
(5)�������������еķ�Һ�����̿�(��Ҫ�ɷ�ΪMnO2)��Ӧ������������(MnSO4��������ˮ)���÷�Ӧ�����ӷ���ʽΪ__________________________________
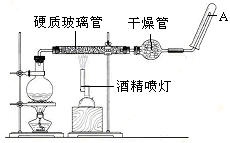
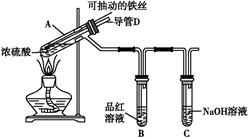
(6)�о����֣���ʯī������������������������CaF2��CaO������ʣ�������ͼ��ʾװ�ÿɻ�ý����ƣ������Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
д�������ĵ缫��Ӧʽ��_________________________
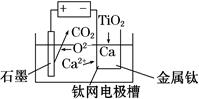
���Ʊ�������ǰ��CaO���������䣬��ԭ����______________________________________(���ϻ�ѧ�������)��

��֪��TiOSO4��ˮ��ˮ�⡣
(1)������У������۽�Fe3��ת��ΪFe2�������ӷ���ʽΪ_______________________
(2)������У�ʵ�ֻ����ķ������������ʵ�________(����ĸ���)��
a���ۡ��е���졡 b���ܽ��Բ��졡 c�������ԡ���ԭ�Բ���
(3)����ڡ��ۡ����У�����Ҫ���еIJ�����________(���������)��
(4)���ϻ�ѧ�����û�ѧƽ�����۽��Ͳ�����н�TiO2��ת��ΪH2TiO3��ԭ����
____________________________________________________________��
(5)�������������еķ�Һ�����̿�(��Ҫ�ɷ�ΪMnO2)��Ӧ������������(MnSO4��������ˮ)���÷�Ӧ�����ӷ���ʽΪ__________________________________
(6)�о����֣���ʯī������������������������CaF2��CaO������ʣ�������ͼ��ʾװ�ÿɻ�ý����ƣ������Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
д�������ĵ缫��Ӧʽ��_________________________

���Ʊ�������ǰ��CaO���������䣬��ԭ����______________________________________(���ϻ�ѧ�������)��
(1)2Fe3����Fe===3Fe2����(2)b��(3)����
(4)��Һ�д���ƽ�⣺TiO2����2H2O??H2TiO3��2H������������ˮϡ�͡����º�ƽ�������ƶ�������H2TiO3��(5)MnO2��2Fe2����4H��=Mn2����2Fe3����2H2O��(6)��2O2����4e��=O2��(��C��2O2����4e��=CO2��)�����Ʊ�Tiʱ��������Ӧ��2CaO 2Ca��O2����2Ca��TiO2
2Ca��O2����2Ca��TiO2 Ti��2CaO���ɴ˿ɼ���CaO����������(���Ʊ�Tiʱ������������Ӧ��2Ca2����4e��===2Ca������������Ӧ��2O2����4e��===O2����2Ca��TiO2
Ti��2CaO���ɴ˿ɼ���CaO����������(���Ʊ�Tiʱ������������Ӧ��2Ca2����4e��===2Ca������������Ӧ��2O2����4e��===O2����2Ca��TiO2 Ti��2CaO���ɴ˿ɼ���CaO����������)
Ti��2CaO���ɴ˿ɼ���CaO����������)
(4)��Һ�д���ƽ�⣺TiO2����2H2O??H2TiO3��2H������������ˮϡ�͡����º�ƽ�������ƶ�������H2TiO3��(5)MnO2��2Fe2����4H��=Mn2����2Fe3����2H2O��(6)��2O2����4e��=O2��(��C��2O2����4e��=CO2��)�����Ʊ�Tiʱ��������Ӧ��2CaO
 2Ca��O2����2Ca��TiO2
2Ca��O2����2Ca��TiO2 Ti��2CaO���ɴ˿ɼ���CaO����������(���Ʊ�Tiʱ������������Ӧ��2Ca2����4e��===2Ca������������Ӧ��2O2����4e��===O2����2Ca��TiO2
Ti��2CaO���ɴ˿ɼ���CaO����������(���Ʊ�Tiʱ������������Ӧ��2Ca2����4e��===2Ca������������Ӧ��2O2����4e��===O2����2Ca��TiO2 Ti��2CaO���ɴ˿ɼ���CaO����������)
Ti��2CaO���ɴ˿ɼ���CaO����������)(1)������У������۽�Fe3��ת��ΪFe2���ķ�Ӧ�����ӷ���ʽΪ2Fe3����Fe=3Fe2����(2)������У���ȴ�ᾧ�����������ڲ�ͬ�¶��µ��ܽ��Բ�����ʵ�ֻ����ķ���ġ�(3)��������ͼ��֪������ڡ��ۡ����о�����еIJ����ǹ��ˡ�(4)�����������Һ�д���ƽ�⣺TiO2����2H2O??H2TiO3��2H������������ˮϡ�͡����º�ƽ�������ƶ�������H2TiO3��(5)��Һ����Ҫ������������������������̿�Ӧ�����ӷ���ʽΪMnO2��2Fe2����4H��=Mn2����2Fe3����2H2O��(6)�ٵ��۵���������������Ӧ��2O2����4e��=O2��(��C��2O2����4e��=CO2��)�����Ʊ�������ʱ��������Ӧ��2CaO 2Ca��O2����2Ca��TiO2
2Ca��O2����2Ca��TiO2 Ti��2CaO�ɴ˿ɼ���CaO���������䡣
Ti��2CaO�ɴ˿ɼ���CaO���������䡣
 2Ca��O2����2Ca��TiO2
2Ca��O2����2Ca��TiO2 Ti��2CaO�ɴ˿ɼ���CaO���������䡣
Ti��2CaO�ɴ˿ɼ���CaO���������䡣
��ϰ��ϵ�д�
�����Ŀ
 Fe2++Cr3+
Fe2++Cr3+
 4CuSO4+2Fe2(SO4)3+2H2O��ij�������ø�ԭ������ͭ���̷��Ĺ�������:
4CuSO4+2Fe2(SO4)3+2H2O��ij�������ø�ԭ������ͭ���̷��Ĺ�������: