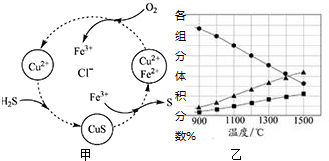
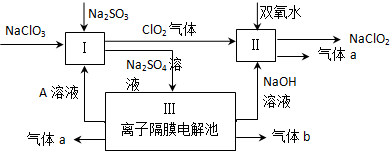
��Ŀ����
3��ij������Һ�п��ܺ��д���Mg2+��Cu2+��Fe3+��K+��H+��NO3-��SO42-��OH-�����е�һ�ֻ��֣���ͨ������ʵ����м��飺��1��ȡ��������Һ����ϸ�۲죬����ɫ��
��2������������Һ�еμ�NaOH��Һ���������������а�ɫ�������ɣ�
��3����������Һ�м���BaCl2��Һ��������
�ݴ˿����жϸô���Һ��һ���������ڵ�������NO3-��Mg2+��H+��һ�����ܴ������ڵ�������Cu2+��Fe3+��SO42-��OH-������ȷ���Ƿ���ڵ�������K+��
���� ��1��ȡ��������Һ����ϸ�۲죬����ɫ������һ��������Cu2+��Fe3+��
��2������������Һ�еμ�NaOH��Һ���������������а�ɫ�������ɣ���һ������Mg2+��H+��һ��������OH-��
��3��������Һ�м���BaCl2��Һ��������һ��������SO42-��
��Һ��ʾ���ԣ�Ӧ�����������Ӿ����ڵģ��ݴ˻ش��жϣ�
��� �⣺��1��ȡ��������Һ����ϸ�۲죬����ɫ������һ��������Cu2+��Fe3+��
��2������������Һ�еμ�NaOH��Һ���������������а�ɫ�������ɣ���һ������Mg2+��H+��һ��������OH-��
��3��������Һ�м���BaCl2��Һ��������һ��������SO42-����Һ��ʾ���ԣ�Ӧ�����������Ӿ����ڵģ�һ������NO3-��
���ϣ�����Һ��һ���������ڵ�������NO3-��Mg2+��H+��һ�����ܴ������ڵ������� Cu2+��Fe3+��SO42-��OH-������ȷ���Ƿ���ڵ�������K+��
�ʴ�Ϊ��NO3-��Mg2+��H+�� Cu2+��Fe3+��SO42-��OH-����K+��
���� ���⿼�������ӹ�������⣬�жϸ���������Һ���ܷ棬��Ҫ����Һ�еĸ�����֮���ܷ�����Ӧ���ɳ��������塢ˮ���ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����������ô�ı䷴Ӧ����������������ͬһ�����ӷ���ʽ��ʾ���������еģ�������
| A�� | �٢ڢ� | B�� | �٢ڢ� | C�� | �٢ۢ� | D�� | �ڢۢ� |
| ���� | ���� | ����� | |
| A | Zn | Al | CuSO4 |
| B | Zn | Cu | CuSO4 |
| C | Cu | Zn | CuCl2 |
| D | Cu | Zn | ZnCl2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | F2��Cl2��Br2��I2����Է��������������۷е������� | |
| B�� | C2Cl6�����и�ԭ�Ӿ�����8�����ȶ��ṹ | |
| C�� | Cl-��S2-��Ca2+��K+�İ뾶��С | |
| D�� | ��������ȵIJ�ͬ���أ�һ�����ڲ�ͬ��Ԫ�� |