��Ŀ����
��2011?�ֶ�����һģ��ij����С��Ϊ��̽��CO2�Ƿ�����ˮͬʱ���ڵ�����²��ܺ�Na2O2�����ͷ�O2�ķ�Ӧ�����������װ�ã������������������жԱ�ʵ�飮

����һ�������CO2��Na2O2��Ӧ
���������ǣ��ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2�����Թܢ���װ���Լ�X��K1��K2��ͨ��CO2���������ǵ�ľ�������Թܢ��Һ���Ϸ����۲�ľ���Ƿ�ȼ���Թܢ��е���ɫ���ޱ仯��
��������������������
���Թܢ��ڼ���Y���������ͬ����һ��
��ش������й����⣺
��1����������ʵ�����ƿ�����
��2��װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2��Ŀ����
��3������һ���Լ�X��
��4���Թܢ��е�NaOH��Һ�������չ�����CO2����ֹ����Ŵ����ǵ�ľ����ȼ����������Ϊ�����Բ��㣬����ˮ��Ҳ����Ÿ�ȼ�����齫��ij���һװ�ã�����Ϊ����ʲôװ�ýϺã���������ķ����н��仭���������������Լ����ƻ�ѧʽ��
��5��Ϊʹʵ����۸�ȷ����ʵ������ȡCO2�������õķ�Ӧ�����ѡ�������е�
a������ʯ b��С�մ� c���ռ� d������ e��ϡ���� f��ϡ���ᣮ

����һ�������CO2��Na2O2��Ӧ
���������ǣ��ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2�����Թܢ���װ���Լ�X��K1��K2��ͨ��CO2���������ǵ�ľ�������Թܢ��Һ���Ϸ����۲�ľ���Ƿ�ȼ���Թܢ��е���ɫ���ޱ仯��
��������������������
���Թܢ��ڼ���Y���������ͬ����һ��
��ش������й����⣺
��1����������ʵ�����ƿ�����
��ʪ��CO2��Na2O2��Ӧ
��ʪ��CO2��Na2O2��Ӧ
����2��װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2��Ŀ����
��ֹNa2O2�ܳ�
��ֹNa2O2�ܳ�
����3������һ���Լ�X��
ŨH2SO4
ŨH2SO4
��������������Y����������ˮ
����ˮ
����4���Թܢ��е�NaOH��Һ�������չ�����CO2����ֹ����Ŵ����ǵ�ľ����ȼ����������Ϊ�����Բ��㣬����ˮ��Ҳ����Ÿ�ȼ�����齫��ij���һװ�ã�����Ϊ����ʲôװ�ýϺã���������ķ����н��仭���������������Լ����ƻ�ѧʽ��
��5��Ϊʹʵ����۸�ȷ����ʵ������ȡCO2�������õķ�Ӧ�����ѡ�������е�
be
be
������ĸ��ţ���a������ʯ b��С�մ� c���ռ� d������ e��ϡ���� f��ϡ���ᣮ
��������1��̽��CO2�Ƿ�����ˮͬʱ���ڵ�����²��ܺ�Na2O2�����ͷ�O2�ķ�Ӧ����������̽����ʪ������̼��������Ʒ�Ӧ��ʵ�飻
��2���ر�K1��K2��Ŀ���Ƿ�ֹ����������ˮ��Ӧ��
��3���ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2�����Թܢ���װ���Լ�X��K1��K2��ͨ��CO2���������ǵ�ľ�������Թܢ��Һ���Ϸ����۲�ľ���Ƿ�ȼ���Թܢ��е���ɫ���ޱ仯�жϣ��Թܢ���װ���Լ�X�Ǹ�������ĸ����Ũ����������� �Լ�����ˮ�������õ���ʪ�Ķ�����̼���壻
��4���Թܢ��е�NaOH��Һ�������չ�����CO2����ֹ����Ŵ����ǵ�ľ����ȼ��Ҳ�������ø�ʢ��ʯ��������չ���������̼��ˮ������
��5����ȡCO2�������õķ�Ӧ�Ӧѡ��ϡ���ᣬ������������ӷ��������ʯ�����������ʣ��ռ�����ɶ�����̼���壮
��2���ر�K1��K2��Ŀ���Ƿ�ֹ����������ˮ��Ӧ��
��3���ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2�����Թܢ���װ���Լ�X��K1��K2��ͨ��CO2���������ǵ�ľ�������Թܢ��Һ���Ϸ����۲�ľ���Ƿ�ȼ���Թܢ��е���ɫ���ޱ仯�жϣ��Թܢ���װ���Լ�X�Ǹ�������ĸ����Ũ����������� �Լ�����ˮ�������õ���ʪ�Ķ�����̼���壻
��4���Թܢ��е�NaOH��Һ�������չ�����CO2����ֹ����Ŵ����ǵ�ľ����ȼ��Ҳ�������ø�ʢ��ʯ��������չ���������̼��ˮ������
��5����ȡCO2�������õķ�Ӧ�Ӧѡ��ϡ���ᣬ������������ӷ��������ʯ�����������ʣ��ռ�����ɶ�����̼���壮
����⣺��1��̽��CO2�Ƿ�����ˮͬʱ���ڵ�����²��ܺ�Na2O2�����ͷ�O2�ķ�Ӧ����������̽�����������̼���������Ƿ�Ӧ��ʵ�飻��������̽����ʪ������̼��������Ʒ�Ӧ��ʵ�飬
�ʴ�Ϊ����ʪ��CO2��Na2O2��Ӧ��
��2���ر�K1��K2��Ŀ���Ƿ�ֹ����������ˮ��Ӧ����ֹ�������Ƴ�ʪ���ʴ�Ϊ����ֹNa2O2�ܳ���
��3����ͨ��CO2֮ǰ���ر�K1��K2�����Թܢ���װ���Լ�X��K1��K2��ͨ��CO2���������ǵ�ľ�������Թܢ��Һ���Ϸ����۲�ľ���Ƿ�ȼ���Թܢ��е���ɫ���ޱ仯�жϣ��Թܢ���װ���Լ�X�Ǹ�������ĸ����Ũ����������� �Լ�����ˮ�������õ���ʪ�Ķ�����̼���壬
�ʴ�Ϊ��ŨH2SO4������ˮ��
��4���Թܢ��е�NaOH��Һ�������չ�����CO2����ֹ����Ŵ����ǵ�ľ����ȼ����������Ϊ�����Բ��㣬����ˮ��Ҳ����Ÿ�ȼ����Ҫ��ȥˮ������ͬʱ��ȥ˯���E���Ͷ�����̼�������Ǽ�ʯ�ң�װ��ͼΪ ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��5����ȡCO2�������õķ�Ӧ�Ӧѡ��ϡ���ᣬ������������ӷ��������ʯ�����������ʣ��ռ�����ɶ�����̼���壬������Ҫѡ��С�մ��ϡ���ᷴӦ���ɣ�
�ʴ�Ϊ��be��
�ʴ�Ϊ����ʪ��CO2��Na2O2��Ӧ��
��2���ر�K1��K2��Ŀ���Ƿ�ֹ����������ˮ��Ӧ����ֹ�������Ƴ�ʪ���ʴ�Ϊ����ֹNa2O2�ܳ���
��3����ͨ��CO2֮ǰ���ر�K1��K2�����Թܢ���װ���Լ�X��K1��K2��ͨ��CO2���������ǵ�ľ�������Թܢ��Һ���Ϸ����۲�ľ���Ƿ�ȼ���Թܢ��е���ɫ���ޱ仯�жϣ��Թܢ���װ���Լ�X�Ǹ�������ĸ����Ũ����������� �Լ�����ˮ�������õ���ʪ�Ķ�����̼���壬
�ʴ�Ϊ��ŨH2SO4������ˮ��
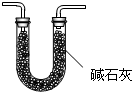
��4���Թܢ��е�NaOH��Һ�������չ�����CO2����ֹ����Ŵ����ǵ�ľ����ȼ����������Ϊ�����Բ��㣬����ˮ��Ҳ����Ÿ�ȼ����Ҫ��ȥˮ������ͬʱ��ȥ˯���E���Ͷ�����̼�������Ǽ�ʯ�ң�װ��ͼΪ
 ��
�� ��
���ʴ�Ϊ��
 ��
�� ��
����5����ȡCO2�������õķ�Ӧ�Ӧѡ��ϡ���ᣬ������������ӷ��������ʯ�����������ʣ��ռ�����ɶ�����̼���壬������Ҫѡ��С�մ��ϡ���ᷴӦ���ɣ�
�ʴ�Ϊ��be��
���������⿼���˹����������ʵķ�������Ӧԭ����̽��������ʵ����̷�����ʵ�������������������ȷ�����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
�����Ŀ