��Ŀ����
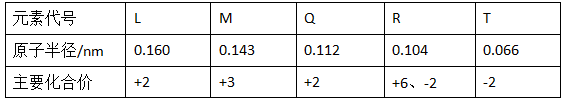
��9�֣�����1���±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
|
Ԫ�ش��� |
L |
M |
Q |
R |
T |
|
ԭ�Ӱ뾶/nm |
0.160 |
0.143 |
0.112 |
0.104 |
0.066 |
|
��Ҫ���ϼ� |
+2 |
+3[ |
+2 |
+6��-2 |
-2 |
��LԪ�ص�������______��RԪ�ص�������______��
������TԪ����ɵĻ�����������Ե���________________��д��ѧʽ����
��2��ij����ѩ����Ҫ�ɷ�ΪXY2��X��Y��Ϊ���ڱ���ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1molXY2����54mol���ӡ�
�ٸ���ѩ����Ҫ�ɷֵĻ�ѧʽΪ__________��X����Ԫ���γɵĻ�����ĵ���ʽΪ____________________��
��Ԫ��D��Eԭ�ӵ���������������Ӧԭ�ӵ��Ӳ�����2����D��Y���ڣ�DԪ����______��EԪ����______��
��1����þ����2�֣� ��BeO��Al2O3 ��2�֣�
��2����CaCl2��1�֣���[H��]-Ca2+[��H]- ��2�֣���S������C����̼����2�֣�
����������1������Ԫ�ص���Ҫ���ϼۺ�ԭ�Ӱ뾶��֪��L��Mg��M��Al��Q��Be��R��S��T��O����������ڶԽ����ϣ��������������Ƶġ�
��2��1molXY2����54mol���ӣ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ���������ӵ�����������18����X��Ca��Y��Cl�����ǻ��õĽ�������������γ����ӻ������⻯�ƣ�����ʽΪ[H��]-Ca2+[��H]- ����������������Ӧԭ�ӵ��Ӳ�����2�����������C��S������ΪD��Y���ڣ�����D��S��E��C��

 ��У����ϵ�д�
��У����ϵ�д���9�֣�����1���±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.112 | 0.104 | 0.066 |
| ��Ҫ���ϼ� | +2 | +3[ | +2 | +6��-2 | -2 |
������TԪ����ɵĻ�����������Ե���________________��д��ѧʽ����
��2��ij����ѩ����Ҫ�ɷ�ΪXY2��X��Y��Ϊ���ڱ���ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1molXY2����54mol���ӡ�
�ٸ���ѩ����Ҫ�ɷֵĻ�ѧʽΪ__________��X����Ԫ���γɵĻ�����ĵ���ʽΪ____________________��
��Ԫ��D��Eԭ�ӵ���������������Ӧԭ�ӵ��Ӳ�����2����D��Y���ڣ�DԪ����______��EԪ����______��
��1���±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.112 | 0.104 | 0.066 |
| ��Ҫ���ϼ� | +2 | +3[ | +2 | +6��-2 | -2 |
��LԪ�ص�������________��RԪ�ص�������________��
������TԪ����ɵĻ�����������Ե���________��д��ѧʽ����
��2��ij����ѩ����Ҫ�ɷ�ΪXY2��X��Y��Ϊ���ڱ���ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1mol XY2����54mol���ӡ�
�ٸ���ѩ����Ҫ�ɷֵĻ�ѧʽΪ________��X����Ԫ���γɵĻ�����ĵ���ʽΪ________��
��Ԫ��D��Eԭ�ӵ���������������Ӧԭ�ӵ��Ӳ�����2����D��Y���ڣ�DԪ����________��EԪ����________��