��Ŀ����
��1�����״����4.48LCO2��������ԭ����Ŀ��ͬ��ˮ�������� g��
��2��V L Fe2��S04��3��Һ�к�Fe3+m g������Һ��SO4 2-�����ʵ���Ũ��Ϊ mol��L��
2-�����ʵ���Ũ��Ϊ mol��L��
��3��9.2g���������NOx���к���ԭ��0.2mol����x����ֵΪ________________��
��4��0.4molij��������Ϊ9.8L����������Ħ�����Ϊ ����������������__________����ǡ����ǡ�����״����
��5�������dz��õ��к�θ���ҩ�

����10Ƭθ��ƽ��5Ƭ��ϲ�����������ʵ����϶���� ��
��ϰ��ϵ�д�
�����Ŀ
���к͵ζ����ⶨij��������ʵ���Ũ�ȡ�
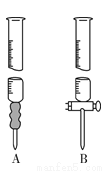
��1������ҺӦʢ��________���A����B�����ζ����С�
��2����ѡ�÷�̪��ָʾ������0.125 0 mol��L��1�ı�����������Һ�ζ�������жϵζ��յ�________________________��
��3��ʵ�����ݼ�¼���±�����������ݲ����㣬��������ʵ���Ũ�ȣ�__________mol��L��1��
�ζ����� | ������Һ���/mL | ||
����Һ | |||
�ζ�ǰ����/mL | �ζ������/mL | ||
1 | 20.00 | 0.00 | 16.02 |
2 | 20.00 | 0.00 | 15.98 |
3 | 20.00 | 0.00 | 16.00 |


 3Z��g�����˷�Ӧ�ﵽƽ��ı�־�ǣ� ��
3Z��g�����˷�Ӧ�ﵽƽ��ı�־�ǣ� ��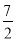 O2��g�� = 2CO2��g����3H2O��g�� ��H����1090 kJ��mol��1
O2��g�� = 2CO2��g����3H2O��g�� ��H����1090 kJ��mol��1 18 mol.L-1��ŨH2SO4��100 mLˮ��ϣ������9 moI.L-l��H2SO4��Һ
18 mol.L-1��ŨH2SO4��100 mLˮ��ϣ������9 moI.L-l��H2SO4��Һ

 ʵ��װ��
ʵ��װ��