��Ŀ����
����Ŀ������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ��������
(1)������ʵ���������ó����йع������Ƹ�ˮ��Ӧ�Ľ�����
a�����������ɣ�b.________���������Ƹ�ˮ��Ӧ�Ļ�ѧ����ʽ_____��
(2)ij�о���ѧϰС��������ͼ��ʾװ�ý���ʵ�飬��֤���������ۡ�
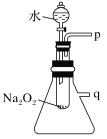
��������֤����a��ʵ�鷽����������____________��
��������֤����b��ʵ�鷽����������___________��
���𰸡���Ӧ���� 2Na2O2+2H2O=4NaOH+O2�� ��p���ռ�һ�Թ����壬�����������ľ���Ž��Թ��У���ľ����ȼ����֤�����ɵ�������O2 ��q���ӵ��ܺ����ˮ�У������ܿ�������ð����֤����Ӧ�ų�����
��������
(1)����������ˮ��Ӧ����O2�����ҷ�Ӧ���ȣ��ﵽ��֬���Ż�㣬ʹ��֬����ȼ�գ��÷�Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
(2)�ٿ���p���ռ����������壬���ô��������ľ�����飻
����q�����ӵ��ܲ����һʢ��ˮ���ձ�������Ӧ���ȣ�����ƿ�������������ͣ�ˮ�л������ݲ�����

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ���ں�����ߺ�DZˮͧ�п��ù���������Ϊ����������ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ������ͼ�е�ʵ��װ�ý���ʵ�飬֤���������ƿ�������������
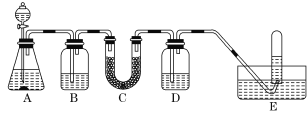
(1)A����ȡCO2��װ�á�д��A�з�����Ӧ�Ļ�ѧ����ʽ��______��
(2)��д���пո�
���� | �����Լ� | ������Լ���Ŀ�� |
B | ����NaHCO3��Һ | ��ȥCO2�л��е�HCl |
C | Na2O2 | _______ |
D | NaOH��Һ | _______ |
(3)д��Na2O2��CO2��Ӧ�Ļ�ѧ����ʽ��______��
(4)�Թ����ռ����������һ��ʵ���������������___��
����Ŀ������˶�Ա����ɵ��ܡ�������Ŀʱ���á�þ�ۡ����֣�������Ч����ij�֡�þ�ۡ��п��ܺ���Mg��MgO��Mg(OH)2��MgCO3�е�һ�ֻ����ֹ��壬ʵ��С�����ɷ�չ����̽����
��֪��MgO+2HCl=MgCl2+H2O MgCO3+2HCl=MgCl2+H2O+CO2��
��1��̽����þ�ۡ����Ƿ���Mg��MgCO3
��ȡ������Ʒ����ͼ��ʾ����ʵ�顣�۲쵽a�Թ��������ݲ�����b�Թ��в���������������ʯ��ˮ����ǣ���֤����þ�ۡ���һ������______��

��Ϊ֤����þ�ۡ����Ƿ���Mg��С����ȼ�ŵ�ľ������ͼ��b�Թܿ��Ϸ���ľ��Ϩ�𡣵�ͬѧ��ָ������ʵ�鲻�ܴ��ʵ��Ŀ�ģ���Ҫ��ͼ�еij���ʯ��ˮ�滻��ŨNaOH��Һ��Ŀ����______________��С�������ĺ��ʵ�鷽���ظ�����ʵ�飬�۲쵽b�Թ�������������֤����þ�ۡ���______________��
��2��̽����þ�ۡ����Ƿ���MgO��Mg(OH)2
��ʵ����̡�
��.��MgO��Mg(OH)2��MgCO3���ֹ���ֱ�������ʵ�顣�ֱ�ȡ0.5g���ֹ����ĩ��ÿ��ȡ��������ͼ2��ʾ��

��μ�����ͬ��������������ϡ����ֱ����ĩǡ����ʧ�����±��м�¼���ĵ�ͬŨ��ϡ���������������������ͬһ�����²ⶨ���ұ�����С�����1λ��
MgO | Mg(OH)2 | MgCO3 | |
����ϡ�������� /mL | 10.4 | 7.2 | 5.0 |
Mg(OH)2�����ᷢ���кͷ�Ӧ�Ļ�ѧ����ʽΪ_____________��
��.ȡ��þ�ۡ���Ʒ0.5 g����������ϡ��������ĩǡ���ܽ⡣��ʱ����ϡ��������ԼΪ5.3 mL��
��ʵ����������ۡ�
��þ�ۡ���ֻ����MgCO3��������____________________________________��
��ʵ�鷴˼��
Ϊȷ����þ�ۡ��ľ���ɷ֣�ͬѧ����Ϊ����Ҫ��������ʵ�飺�ֱ�ȡ0.5 g��þ�ۡ���0.5 g_________����������ϡ���ᣬ�ⶨ���ɵ���������ֱ�Ϊ119mL��140mL���ɴ˿�֪��þ�ۡ��к���MgCO3����������Ϊ___________��




