��Ŀ����
ijѧϰС���̿������������Ӧ����������ijɷֽ����о���
��1��������裺�÷�Ӧ���������ȫ���Ƕ�����̼��CO2����
��2����Ʒ�������һ��������������̿�۵Ļ�����ڸ�����������������ȫ��Ӧ������ͼ�����ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�

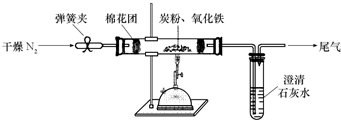
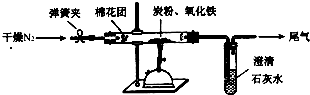
ʵ��װ��
��3���������ϣ�N2����̿���Լ�������������Ӧ�������ڸ����������з�Ӧ��
��4��ʵ�������
�ٳ�ȡ5.2 g��������̿�۵Ļ�Ϸ�ĩ������48.48 g�IJ������У�����ͼ���Ӻ�ز����ٵ�ʵ�����Ϊ___________________________________________________��
�ڼ���ǰ����ͨһ��ʱ�䴿��������ĵ�������Ŀ����_____________________________��
�ۼн�T�����ɼУ�����һ��ʱ�䣬����ʯ��ˮ����ǣ�������˵��_____________________��
����ȫ��Ӧ����ȴ�����£��Ƶò����ܺ����������Ϊ52.24�ˡ�
��5�����ݴ����������㣬�μӷ�Ӧ��̼Ԫ������Ϊ0.48�ˣ���Ԫ������Ϊ0.96�ˡ�
��6���ó����ۣ��������ݴ����������Ӧ���ɵ�����ΪCO��CO2������ͬ�����������Ϊ1��1��ԭ���費����������Ϊ__________________________________________________��
��7����ʵ�鷴Ӧ�Ļ�ѧ����ʽΪ_________________________________________________��
��4���ټ��װ�������� ���ž�װ���еĿ������������� ���ж�����̼��������
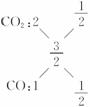
��6��C��O������Ϊ0.48��0.96=1��2���������̼��C��O������3��8����
��7��2C+Fe2O3![]() 2Fe+CO��+CO2��
2Fe+CO��+CO2��
����:
�������⣬��ʵ��Ϊһ����ʵ�飬��ԭ���ǽ�һ����Fe2O3��C�Ļ��������N2������������������ȫ��Ӧ����μӷ�Ӧ��CԪ�غ�OԪ�ص������ȣ��жϷ�Ӧ�����Ƿ�ȫ��CO2����˷�Ӧװ�ñ��������Ժá�
�μӷ�Ӧ��CԪ������Ϊ0.48 g�����ʵ���Ϊ0.04 mol��OԪ�ص�����Ϊ0.96 g�����ʵ���Ϊ0.06 mol�����ɵ�̼��������C��O���ʵ���֮��Ϊ2��3,��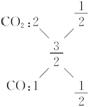 ����CO��CO2�����ʵ�����Ϊ1��1�����ԭ���費��������ʵ�鷴Ӧ�Ļ�ѧ����ʽΪ2C+Fe2O3
����CO��CO2�����ʵ�����Ϊ1��1�����ԭ���費��������ʵ�鷴Ӧ�Ļ�ѧ����ʽΪ2C+Fe2O3![]() 2Fe+CO��+CO2����
2Fe+CO��+CO2����


 ij�о���ѧϰС��Թ���̿������������Ӧ���������ɷֽ����о���
ij�о���ѧϰС��Թ���̿������������Ӧ���������ɷֽ����о���