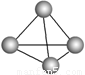
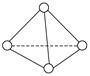
��Ŀ����
��֪����1 mol C��H����Ҫ��������414.4 kJ������1 mol C��C����Ҫ��������347.4 kJ������1 mol C===C������ų�����615.3 kJ������1 mol H��H������ų�����435.3 kJ��ij�л���ֽ�ķ�Ӧ�ɱ�ʾΪ��
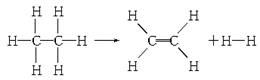
���ڷ�Ӧ��������1 mol���飬���йظ÷�Ӧ��˵����ȷ����
A���÷�Ӧ�ų�251.2 kJ������ B���÷�Ӧ����251.2 kJ������
C���÷�Ӧ�ų�125.6 kJ������ D���÷�Ӧ����125.6 kJ������
���𰸡�
D
����������Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ�����Ը��ݼ��ܿ�֪����Ӧ���ǡ�H��414.4 kJ/mol��6��347.4 kJ/mol��414.4 kJ/mol��4��615.3 kJ/mol��435.3 kJ/mol����125.6 kJ/mol�����Դ�ѡD��

��ϰ��ϵ�д�
�����Ŀ
����п�ѧ�һ���˼��������о������N4���ӡ�N4���ӽṹ����ͼ��ʾ����֪����1 mol N��N����167 kJ����������1 mol N  N�ų�942kJ������������Ϣ�����ݣ�����˵����ȷ����
N�ų�942kJ������������Ϣ�����ݣ�����˵����ȷ����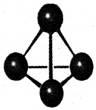
| A��N4����һ�����͵Ļ����� |
| B��N4��N2��Ϊͬ�������� |
| C��N4���γɵľ�������ԭ�Ӿ��� |
| D��N4����ת��ΪN2���������� |
 N�ų�942 kJ����������������Ϣ�����ݣ��ж�����˵����ȷ����
N�ų�942 kJ����������������Ϣ�����ݣ��ж�����˵����ȷ����