��Ŀ����
������һ����Ҫ�Ļ�ѧ�Լ���ijʵ��С���ͬѧ����Ũ���������ȡ��������̽�������ʵ�ʵ�飮�밴Ҫ��ش��������⣮
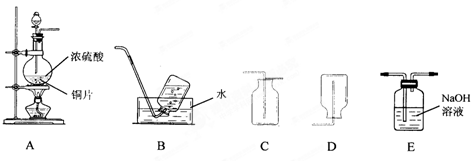
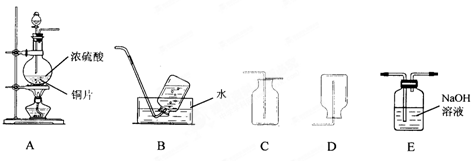
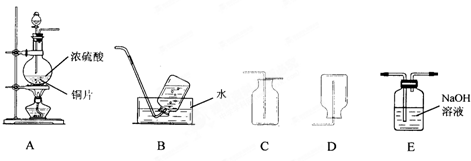
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ______������װ���������ռ���������������ǣ�����ĸ��______��
��2����һ�ռ����������������С�Թܵ����ڵ�����ɫʯ����Һ��ˮ�У��ɹ۲쵽��������______��
��3������ʵ���������Ķ�������β����ѡ��Eװ�������գ��÷�Ӧ�Ļ�ѧ����ʽΪ______��

��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ______������װ���������ռ���������������ǣ�����ĸ��______��
��2����һ�ռ����������������С�Թܵ����ڵ�����ɫʯ����Һ��ˮ�У��ɹ۲쵽��������______��
��3������ʵ���������Ķ�������β����ѡ��Eװ�������գ��÷�Ӧ�Ļ�ѧ����ʽΪ______��
��1������װ��ͼ���Լ����������ʷ�����Ũ�����ͭ���ȷ�Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4��Ũ��
CuSO4+2H2O+SO2�������ݶ�����������������ˮ������B���ȿ����ز�����D����Ҫ�������ſ������ռ�������ѡC��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2����C��
��2����һ�ռ����������������С�Թܵ����ڵ�����ɫʯ����Һ��ˮ�У�������������ˮ��ˮ��Ӧ���������ᣬˮ������������������ǿ�ᣬ��ʯ���죬
�ʴ�Ϊ���Թ���Һ����������Һ��ɺ�ɫ��
��3��������������Ⱦ�����壬�ŷŵ���������Ⱦ��������Ҫ����������Һ���գ���Ӧ�����������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+SO2=Na2SO3+H2O��
�ʴ�Ϊ��2NaOH+SO2=Na2SO3+H2O��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��2����һ�ռ����������������С�Թܵ����ڵ�����ɫʯ����Һ��ˮ�У�������������ˮ��ˮ��Ӧ���������ᣬˮ������������������ǿ�ᣬ��ʯ���죬
�ʴ�Ϊ���Թ���Һ����������Һ��ɺ�ɫ��
��3��������������Ⱦ�����壬�ŷŵ���������Ⱦ��������Ҫ����������Һ���գ���Ӧ�����������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+SO2=Na2SO3+H2O��
�ʴ�Ϊ��2NaOH+SO2=Na2SO3+H2O��

��ϰ��ϵ�д�
�����Ŀ