��Ŀ����
����Ŀ��������A���緢������ţ���У������������Ǵ�л���м��壬�����������������۵ȷ����Ƶá�A�ĸ���������ϲ���IJ��Ƽ�֮һ��A��ij�ִ����Ĵ����±�����������ﲻ�ܷ���������Ӧ����Ũ��������£� A�ɷ���������ʾ�ķ�Ӧ��

��1��A���еĹ���������Ϊ_____________________,A��F�ķ�Ӧ����Ϊ __________��
��2��д��һ�ֺ�B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ___________________________��
��3��д��A��D�Ļ�ѧ����ʽ_______________________________________________________��
��4���л���C3H6O����ȩ����һ�������º�H2��Ӧ����CH3CH2CH2OH��������л����Ƿ���ȫת���IJ�����_______________________________________________________________��
��5���л���E������CH2=CHCH2OH�õ����ı�������Ҳ�����Ƶ�����������һ����CH2=CHCH2OH�ϳ�CH3CH2COOH��·��_____________��
�ϳ�·������ͼʾ�����£�CH3CH2OH![]() CH2��CH2
CH2��CH2![]()
![]()
���𰸡� �ǻ����Ȼ� ȡ�� CH3CH(OH)COOCH2CH3 ����������ͬ�����ŵ�ͬ���칹�� CH3CH(OH)COOH+CH3COOH ![]() CH3CH(COOH)OOCCH3+H2O ȡ������������������ͭ����У��۲��Ƿ���ש��ɫ�������ɣ�����ש��ɫ�������ɣ���δȫ��ת������û��ש��ɫ�������ɣ�����ȫ��ת�� CH2=CHCH2OH
CH3CH(COOH)OOCCH3+H2O ȡ������������������ͭ����У��۲��Ƿ���ש��ɫ�������ɣ�����ש��ɫ�������ɣ���δȫ��ת������û��ש��ɫ�������ɣ�����ȫ��ת�� CH2=CHCH2OH![]() CH3CH2CH2OH
CH3CH2CH2OH![]() CH3CH2COOH
CH3CH2COOH
��������A��Ũ��������¼��ܺ��Ҵ���Ӧ�����ܺ����ᷴӦ��˵��A�м����Ȼ������ǻ���A�������IJ��ﲻ�ܷ���������Ӧ��˵���ǻ�����̼���Ķ˵��ϣ����ж�AΪ����CH3CH��OH��COOH����A�������ɵ�CH3COCHO�����ܷ���������Ӧ����ͽ�һ��֤����A��������A���Ҵ�����������Ӧ����B��BΪCH3CH��OH��COOCH2CH3��A�����ᷢ��������Ӧ����D��DΪCH3COOCH��CH3��COOH��A��Ũ���ᡢ��������������E��E������ˮ��ɫ��Ӧ������ȥ��Ӧ��EΪCH2=CHCOOH��A��Ũ���ᡢ����������������ԭ�ӻ�״������F�����F�ķ���ʽ��֪��Ϊ2�������ᷢ��������Ӧ���ɻ�״�������FΪ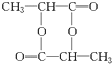 ����1��AΪCH3CH��OH��COOH�������ǻ����Ȼ���A��F��������Ũ���ᡢ���������·���������Ӧ���ɻ�״������
����1��AΪCH3CH��OH��COOH�������ǻ����Ȼ���A��F��������Ũ���ᡢ���������·���������Ӧ���ɻ�״������ ����Ӧ����ʽΪ
����Ӧ����ʽΪ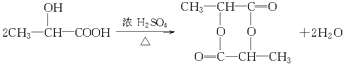 ����Ӧ����Ϊȡ����Ӧ����2��BΪCH3CH��OH��COOCH2CH3��B������ͬ�����ŵ�ͬ���칹�庬���ǻ������������������Ľṹ��ʽ��CH3CH(OH)COOCH2CH3����3��A��D��CH3CH��OH��COOH��CH3COOH��Ũ������·���������Ӧ����CH3COOCH��CH3��COOH��ˮ����Ӧ�Ļ�ѧ����ʽCH3CH(OH)COOH+CH3COOH
����Ӧ����Ϊȡ����Ӧ����2��BΪCH3CH��OH��COOCH2CH3��B������ͬ�����ŵ�ͬ���칹�庬���ǻ������������������Ľṹ��ʽ��CH3CH(OH)COOCH2CH3����3��A��D��CH3CH��OH��COOH��CH3COOH��Ũ������·���������Ӧ����CH3COOCH��CH3��COOH��ˮ����Ӧ�Ļ�ѧ����ʽCH3CH(OH)COOH+CH3COOH ![]() CH3CH(COOH)OOCCH3+H2O����4���л���C3H6O����ȩ����һ�������º�H2��Ӧ����CH3CH2CH2OH��������л����Ƿ���ȫת���IJ�����ȡ������������������ͭ����У��۲��Ƿ���ש��ɫ�������ɣ�����ש��ɫ�������ɣ���δȫ��ת������û��ש��ɫ�������ɣ�����ȫ��ת������5��CH2=CHCH2OH����������CH3CH2CH2OH��CH3CH2CH2OH�����õ�CH3CH2COOH���ϳ�CH3CH2COOH��·��Ϊ��CH2=CHCH2OH
CH3CH(COOH)OOCCH3+H2O����4���л���C3H6O����ȩ����һ�������º�H2��Ӧ����CH3CH2CH2OH��������л����Ƿ���ȫת���IJ�����ȡ������������������ͭ����У��۲��Ƿ���ש��ɫ�������ɣ�����ש��ɫ�������ɣ���δȫ��ת������û��ש��ɫ�������ɣ�����ȫ��ת������5��CH2=CHCH2OH����������CH3CH2CH2OH��CH3CH2CH2OH�����õ�CH3CH2COOH���ϳ�CH3CH2COOH��·��Ϊ��CH2=CHCH2OH![]() CH3CH2CH2OH
CH3CH2CH2OH![]() CH3CH2COOH��
CH3CH2COOH��

����Ŀ�����㻯�����ڴ���������±��������ȡ����Ӧ��ΪFriedel-Crafts�������Ӧ��ij����С���Ա����ȴ��嶡��[ClC(CH3)3]Ϊ��Ӧ���ˮAlCl3Ϊ�����������Ʊ��嶡����(![]() )��
)��
��Ӧ����: +ClC(CH3)3
+ClC(CH3)3 +HCl
+HCl
��֪������Ϣ:
���� | ��Է������� | �ܶ� | �۵� | �е� | �ܽ��� |
AlCl3 | ���� | ���� | 190�� | 180�� | ��ˮ���׳��Ⲣ������ɫ���������ڱ� |
�� | 78 | 0.88g/cm3 | ���� | 80.1�� | ������ˮ���������Ҵ� |
�ȴ��嶡�� | 92.5 | 1.84 g/cm3 | ���� | 51.6�� | ������ˮ�������ڱ� |
�嶡���� | 134 | 0.87 g/cm3 | ���� | 169�� | ������ˮ�������ڱ� |
I������ͼ��ʵ�����Ʊ���ˮAlCl3��ʵ��װ��:

(1)Eװ���е�����������_______________��
(2)д��Bװ���з�����Ӧ�����ӷ���ʽ:________________________��
(3)ѡ����ʵ�װ���Ʊ���ˮAlCl3��ȷ������˳��Ϊ:_____________ (д���ܿڱ��)��
(4)���в�����ȷ����________��
��:�ȼ���Ӳ�ʲ������ټ���Բ����ƿ
��:�ȼ���Բ����ƿ�ټ���Ӳ�ʲ�����
(5)Eװ�õ�������:_______________________��
II��ʵ������ȡ�嶡����װ����ͼ:

��������ƿ�м���50mL�ı�����������ˮAlCl3���ɺ�ѹ©���μ��ȴ��嶡��[ClC(CH3)3]10mL��һ���¶��·�Ӧһ��ʱ�����Ӧ��Ļ����ϴ�ӷ��룬�����ò����м���������ˮMgSO4���壬���ã����ˣ�������嶡����20g��
(6)ʹ�ú�ѹ©�����ŵ���____________________������ˮMgSO4�����������___________��
(7)������Ӧ�������ϴ�����õ��Լ����������֣���ȷ��˳����_____________��
��5%Na2CO3��Һ ��ϡ���� ��H2O
(8)�嶡�����IJ���Ϊ______��(����3λ��Ч����)
���𰸡� ����� MnO2+4H++2C1-=Mn2++Cl2��+2H2O d��e��f��g��h��i��j��c �� ��ֹ�����е�ˮ�������룬�����ն�������� ʹҺ��˳������ ���� �ڢ٢� 75.0%
��������I.(1). Eװ���е����������Ǹ���ܣ��ʴ�Ϊ������ܣ�
(2). ��Bװ������Ũ�����MnO2��Ӧ�����Ȼ��̡�������ˮ�����ӷ���ʽΪ��MnO2+4H++2Cl=Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl=Mn2++Cl2��+2H2O��
(3). Bװ�ò����������л����Ȼ����ˮ�������ʣ���Dװ�ó�ȥHCl ������Cװ�ó�ȥˮ�������ʣ������Ȼ������������������Ȼ����������������ܣ����Եõ���������������Fװ���к�����Ӧ��ȡ�Ȼ���������Aװ�ã�������Ϣ��֪�Ȼ�����ˮ���׳��Ⲣ������ɫ������������������Eװ�����ն�������������Է�ֹ�����е�ˮ��������Fװ���У�����Ʊ���ˮ�Ȼ�����ȷ������˳��Ϊd��e��f��g��h��i��j��c���ʴ�Ϊ��d��e��f��g��h��i��j��c��
(4). �Ʊ���ˮ�Ȼ���ʱ��Ӧ�ȼ���Բ����ƿ������������װ���еĿ����ž����Է�ֹ���۱������е������������ʴ�Ϊ���ң�
(5). ������������֪����Eװ�ÿ������ն�����������ܷ�ֹ�����е�ˮ��������Fװ����ʹ�Ȼ������⣬�ʴ�Ϊ����ֹ�����е�ˮ�������룬�����ն����������
II. (6). ʹ�ú�ѹ©������ƽ��©������ѹǿ��ʹҺ��˳�����£���ϴ�Ӻ����ò����м���������ˮMgSO4������Ŀ�������ղ�Ʒ��������ˮ�֣���������ã��ʴ�Ϊ��ʹҺ��˳�����£����
(7). ϡ����ϴ�ӿ��Գ�ȥ�Ȼ������ʣ�����5%Na2CO3��Һ��ȥ���������ᣬ�����ˮϴ�ӳ�ȥʣ���5%Na2CO3���ʴ�Ϊ���ڢ٢ۣ�
(8).���뱽�����ʵ���Ϊ50mL��0.88g/mL��78g/mol=0.56mol���ȴ��嶡������ʵ���Ϊ10mL��1.84g/mL��92.5g/mol=0.199mol���ɷ�Ӧ����ʽ +ClC(CH3)3
+ClC(CH3)3 +HCl��֪������ı��������������������嶡����������Ϊ��0.199mol��134g/mol=26.66g���嶡�����IJ���Ϊ��
+HCl��֪������ı��������������������嶡����������Ϊ��0.199mol��134g/mol=26.66g���嶡�����IJ���Ϊ��![]() ��100%=75.0%���ʴ�Ϊ��75.0%��
��100%=75.0%���ʴ�Ϊ��75.0%��
�����͡�ʵ����
��������
9
����Ŀ��һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3��CoO����ʽ���ڣ������������ĵ����˫�棺﮻��������С�(��֪Co2O3��������>Cl2��������)�ӷ����л���������(CoO)�Ĺ����������£�

��֪����CoCO3���ܶȻ�Ϊ��Ksp=1.0��10-13��
����Һ������Ũ��С��1.0��10-5mol/Lʱ��Ϊ�����ӳ�����ȫ��
(1)�����ܡ�ǰͨ�������Ϸ��飬��Ŀ����____________��
(2)����I�в���NaOH��Һ�ܳ������е�A1����Ӧ�����ӷ���ʽΪ_________________��
(3)���̢��м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܡ�������������ʵķ�Ӧ��ѧ����ʽΪ (������ֻ��һ�����) _______________________________________����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ�����������������ܵ���Ҫԭ��______________________________________��
(4)����III�õ����������Ҫ�ɷ���LiF��AI(OH)3��̼������Һ�ڲ��� Al(OH)3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ__________________________________��
(5)��2.0��10-4 mol/LCoSO4��2.2��10-4mol/L��Na2CO3�������ϣ���ʱ��Һ�е�Co2+��Ũ��Ϊ__________��Co2+�Ƿ������ȫ? __________(��ǡ���)��
(6)CoO��������ɵ÷ۺ�ɫ��CoCl2��Һ��CoCl2���ᾧˮ��Ŀ��ͬ�����ֲ�ͬ��ɫ��������ɫ����ˮCoCl2��ˮ��ɫ��һ���ʿ��Ƴɱ�ɫˮ�������īˮ����ͼ�Ƿۺ�ɫ��CoCl2��6H2O�������ȷֽ�ʱ��ʣ������������¶ȱ仯�����ߣ�����B�Ļ�ѧʽ��____________________��