��Ŀ����
��ҵ�Ӵ���������ļ�����ͼ���£�
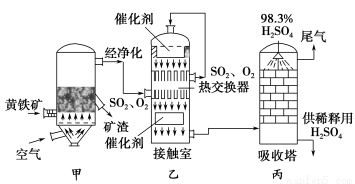
(1)д��װ�ü�����________��Ҫʹ�������ֺ�Ѹ�ٵ�ȼ�գ���ҵ�ϳ���ȡ�Ĵ�ʩΪ__________________________________��
(2)�Ӵ����ж��������������ķ�Ӧ����________(������ѹ��������ѹ��)��ԭ����________________________________��
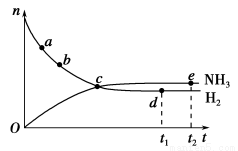
(3)��ͨ��Ӵ����е�SO2��O2���Ƚ������Ĺܵ�________(����������������)�������ڴ�������Ӵ���Ӧ��SO2��O2���Ƚ������Ĺܵ�________(����������������)������������������ͨ���ܱڽ����Ƚ�����
(4)����������Ϊʲô��98.3%��Ũ�����������ˮ������������
______________________________________________________________��
(5)��ҵ�Ӵ���������Ĺ����У��������������̲�����������ԭ�������Ƿֱ���_____________________________________
(1)����¯���ѻ���������ϸС�Ŀ����ʹ�¯��ͨ��ǿ��Ŀ�������(2)��ѹ����ѹʱSO2��ת�����Ѿ��ܸߣ����ø�ѹ��SO2��ת������߲���ȴ���������豸�ɱ���(3)�⡡�(4)ˮ��SO3��Ӧ�ų��������ȣ������γ�������������SO3���ա�(5)�ڷ���¯�п�ʯ���������䣬��������������������������98.3%Ũ�������϶�������SO3����������������
�������������

��Ϊ��������ʵʩ�������������������յ�վ�����Ϻ����ı���ú��ʯ��Ϊ������Դ�ṹ����Խ�����л�����Ⱦ�����ش�
(1)Ŀǰ�Ϻ��ֳ��о�����ʹ�õ�ȼ����Ҫ�ǹܵ�ú�����ֶ���������
��ʼʹ�ö�����Ȼ����Ϊ����ȼ�ϣ��ܵ�ú������Ҫ�ɷ���CO��H2������
���࣬��Ȼ������Ҫ�ɷ���CH4������ȼ�յĻ�ѧ����ʽΪ��
2CO��O2 2CO2��2H2��O2
2CO2��2H2��O2 2H2O��CH4��2O2
2H2O��CH4��2O2 CO2��2H2O
CO2��2H2O
�������ϻ�ѧ����ʽ�жϣ�ȼ����ͬ����Ĺܵ�ú������Ȼ�������Ŀ�������ϴ�����������������ȼ�չܵ�ú����������������Ȼ������ߵĸĽ������������������(����������������С��)���粻���Ľ����ܲ����IJ����������������������������������������
(2)�ܵ�ú���к��е����࣬�������⣬�����������顢���顢����ȣ����ǵ�ijЩ�������£�
| ���� | ���� | ���� |
�۵�/�� | ��183.3 | ��189.7 | ��138.4 |
�е�/�� | ��88.6 | ��42.1 | ��0.5 |
�Ը�������ij���ؼ����ݽ��Ͷ����Ϻ��ļ�����ʱ�ܵ�ú�������С�����ҳʶ���״̬��ԭ����___________________________��