��Ŀ����
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�-36�棬�е�114�棬���������۵�Ϊ231�档װ��A�з�Ũ����B�з�MnO2�����������������������ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ������˷�Ӧ���̷ų��������ȣ�����ش����и����⡣

��1������ͼ�����巢����β������װ�ò������ƣ���������Ľ����_______________________________��
���øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
��2��H�з�Ӧ�����ӷ���ʽ��_________________________________________________��
E�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��3��C��D�е��Լ��ֱ���_______________��____________________��
��4������A��B�����Ʒֱ���_____________��____________��F��������_____________��
��5��ʵ��ʱӦ�ȵ�ȼ_________���ƾ��ƣ������¶�Ӧ����________ �棬��________������ֹͣ���ȡ�
��6����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________________________________________________��
��7���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������___________________________��

��1������ͼ�����巢����β������װ�ò������ƣ���������Ľ����_______________________________��
���øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
��2��H�з�Ӧ�����ӷ���ʽ��_________________________________________________��
E�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��3��C��D�е��Լ��ֱ���_______________��____________________��
��4������A��B�����Ʒֱ���_____________��____________��F��������_____________��
��5��ʵ��ʱӦ�ȵ�ȼ_________���ƾ��ƣ������¶�Ӧ����________ �棬��________������ֹͣ���ȡ�
��6����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________________________________________________��
��7���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������___________________________��
��
��1���õ��ܽ�A���Ͽں�B��������A���ɺ�ѹ��Һ©����1�֣���G��H֮�����Ӹ���װ�ã�1�֣�
��2��Cl2��H2O=Cl�D��ClO�D��H2O��2�֣�Sn��2Cl2 SnCl4��2�֣�
SnCl4��2�֣�
��3������ʳ��ˮ����ˮ����1�֣�Ũ���ᣨ1�֣�
��4����Һ©����1�֣�������ƿ��1�֣�������������2�֣�
��5��E��1�֣�231�棨1�֣�Sn���ۻ���1�֣�
��6��SnCl4��2H2O=SnO2��4HCl��2�֣�
��7�����ְ�ɫ������1�֣�
��1���õ��ܽ�A���Ͽں�B��������A���ɺ�ѹ��Һ©����1�֣���G��H֮�����Ӹ���װ�ã�1�֣�
��2��Cl2��H2O=Cl�D��ClO�D��H2O��2�֣�Sn��2Cl2
 SnCl4��2�֣�
SnCl4��2�֣���3������ʳ��ˮ����ˮ����1�֣�Ũ���ᣨ1�֣�
��4����Һ©����1�֣�������ƿ��1�֣�������������2�֣�
��5��E��1�֣�231�棨1�֣�Sn���ۻ���1�֣�
��6��SnCl4��2H2O=SnO2��4HCl��2�֣�
��7�����ְ�ɫ������1�֣�
�����������1������װ�����������壬ѹǿ����Һ©���е������˳���ӵ�Բ����ƿ�У�Ӧ�õ��ܽ�A���Ͽ���B��������ƽ���Һ©����Բ����ƿ�е�ѹǿ�����Ȼ�������ˮ�⣬GΪ�ռ����Ȼ���װ�ã�Ӧ��G��H֮�����Ӹ���װ�÷�ֹH�е�ˮ��������Gװ�ã���Ϊ���õ��ܽ�A���Ͽ���B��������G��H֮�����Ӹ���װ�ã�
��2��HΪ����Ϊ��Ӧ���������������������Ʒ�Ӧ�����Ȼ��ơ��������ơ�ˮ����Ӧ���ӷ���ʽΪ��Cl2+2OH��=Cl��+ClO��+H2O����װ��ͼ��֪��E��Ϊ�������������������Ӧ������ˮ���Ȼ�������Ӧ����ʽΪ��Sn+2Cl2
 SnCl4������Cl2+2OH��=Cl��+ClO��+H2O��Sn+2Cl2
SnCl4������Cl2+2OH��=Cl��+ClO��+H2O��Sn+2Cl2 SnCl4��
SnCl4����3�����Ȼ�������ˮ�⣬����װ��E������Ӧ���������װ��C������Ϊ���ջӷ�����HCl���ñ���ʳ��ˮ����HCl��װ��D������Ϊ����ˮ�������������壬��Ũ�������ո����Ϊ������ʳ��ˮ��Ũ���
��4������AΪ��Һ©����BΪ������ƿ��F���������������Ȼ�����������Ϊ����Һ©����������ƿ��������������
��5�����ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ�������Ӧ�����E�ƾ��ƣ������¶�Ӧ���ڽ��������۵㣬�����ڻ���ֹͣ���ȣ���Ϊ��E��231�����ڻ���
��6�����Ȼ�����ˮǿ��ˮ�⣬��ˮ��ԭ����֪��Ӧ����Sn(OH)4��HCl������֮һ�ǹ�̬����������˵��Sn(OH)4�ֽ�����SnO2��H2O�������Ȼ���ˮ������SnO2��HCl����Ӧ����ʽΪ��SnCl4+2H2O=SnO2+4HCl����Ϊ��SnCl4+2H2O=SnO2+4HCl��
��7�����Ȼ�����ˮǿ��ˮ������SnO2��HCl��SnO2�ǹ��������HCl��Ͽ����е�ˮ���������ְ�ɫ��������Ϊ�����ְ�ɫ������

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ

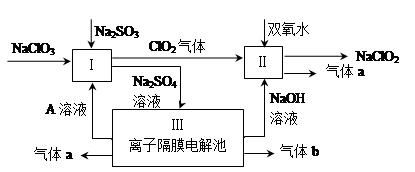

 HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________��
HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________��