��Ŀ����
 ijͬѧ��ѧϰ�����ἰ���ε�ijЩ��������;���У���������ʵ��̽����
ijͬѧ��ѧϰ�����ἰ���ε�ijЩ��������;���У���������ʵ��̽������ʵ��һ��̽��Ũ�����������
��ʵ���ҳ��õ�ҩƷ��������ͼ��ʾ��ʵ��װ�ú���װ�üף����гֺͼ���װ��ʡ�ԣ�
��1����װ������ϴ�������ȱ�ݣ���ָ����
��2��д��װ�ü��з�Ӧ�Ļ�ѧ����ʽ
| ||
 CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O��
| ||
 CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O����3��װ�����е��Լ���
��ʵ�����̽��ij���������ι����Ƿ����
��4�����������ͬѧ�������ʵ�鷽����
| ʵ����� | Ԥ������ͽ��� |
�������ϵ�֪����ҵ���õ��KHSO4������Һ��ȡH2O2��ʾ��ͼ��ͼ��

��ͬѧ�ô˷���ȡһ��Ũ�ȵ�H2O2������������ʵ��ⶨH2O2������������
��ȡ5.00mL H2O2��Һ���ܶ�Ϊ1.00g/mL��������ƿ�м�ˮϡ�ͣ��ټ�ϡ�����ữ��
����0.1000mol/L KMnO4��Һ�ζ���
����ͬ�������ζ�����������KMnO4��Һ������ֱ�Ϊ20.00mL��19.98mL��20.02mL��
�ش��������⣺
��5����ⱥ��KHSO4��Һʱ�������ĵ缫��ӦʽΪ
��6���������У������һ��KMnO4��Һ����Һ�Ϻ�ɫ��ʧ���������ŵζ�������Mn2+�����࣬��Һ�Ϻ�ɫ��ʧ���ʼӿ죮Mn2+��������
��7��д���õζ���Ӧ�����ӷ���ʽ��
��8��ԭH2O2��Һ�����ʵ���������Ϊ
��2��װ���ǹ����Ũ����ķ�Ӧ���Դ���ȷ��Ҫд�ķ�Ӧ����ʽ��
��3�����������������Һ���������ƻ����������أ�
��4�����������е����������ױ�����Ϊ���������ӣ�����������������������������ӣ�
��5�����ص������������ӷ���ʧ���ӵ�������Ӧ��
��6����˫��ˮ�ķֽ�����У����������������ã�
��7���������������ǿ�����ԣ����Խ�˫��ˮ������
��8�����ݷ�Ӧ��ԭ������ʽ���м��㣮
�ʴ�Ϊ��װ���Ҳ�Ӧ��ƿ����
��2��װ���ǹ����Ũ����ķ�Ӧ������ͭ���ǽ�������C�Ⱦ����Ժ�Ũ���ᷴӦ����Cu+2H2SO4��Ũ��
| ||
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
| ||
��3��β�����������������Һ���������ƻ����������أ��ʴ�Ϊ������������Һ��������������Һ�ȣ���
��4���������������ױ�����Ϊ���������ӣ�������������������飬��������������һ֧�Թ��м����������壬��ˮ�ܽ⣬�ٵμӼ���KSCN��Һ������Һ��Ϊ��ɫ��˵���ù����ѱ��ʣ�����Һδ���ɫ��˵���ù���û�б��ʣ�
�ʴ�Ϊ��������������һ֧�Թ��м����������壬��ˮ�ܽ⣬�ٵμӼ���KSCN��Һ������Һ��Ϊ��ɫ��˵���ù����ѱ��ʣ�����Һδ���ɫ��˵���ù���û�б��ʣ�
��5����ⱥ��KHSO4��Һʱ�������������ӷ���ʧ���ӵ�������Ӧ���缫��ӦʽΪ2HSO4--2e-=S2O82-+2H+����2SO42--2e-=S2O82-�����ʴ�Ϊ��2HSO4--2e-=S2O82-+2H+����2SO42--2e-=S2O82-����
��6����˫��ˮ�ķֽ�����У�������ؾ��������ԣ���Ӧ�Ļ�ԭ�����������ӣ��������һ��KMnO4��Һ����Һ�Ϻ�ɫ��ʧ���������ŵζ�������Mn2+�����࣬��Һ�Ϻ�ɫ��ʧ���ʼӿ죬�ɼ������������������ã�
�ʴ�Ϊ����������ӿ췴Ӧ���ʣ���
��7���������������ǿ�����ԣ����Խ�˫��ˮ������ԭ������ʽΪ��2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����
�ʴ�Ϊ��2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����
��8����������KMnO4��Һ������ֱ�Ϊ20.00mL��19.98mL��20.02mL�������ƽ��ֵΪ��20.00mL�������ĸ������������0.1mol/L��0.02L=0.002mol����˫��ˮ�����ʵ���Ϊn����
2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2 5
0.002mol n
���n=0.005mol������˫��ˮ������Ϊ��0.005mol��34g/mol=0.17g��˫��ˮ����������=
| 0.17g |
| 5.00mL��1.00g/mL |

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�. ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���
������ȡ��������Ȫ�����̽��
1.д��ʵ����ȡ�����Ļ�ѧ����ʽ ��
2.�ռ������ķ����� ��
3.��Ȫ��һ�ֳ�������Ȼ����
��1��ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�á���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ͼ3��ʾ��װ�á�
����ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����__________��
A.Cu��ϡ���� B.NaHCO3��NaOH��Һ C.MnO2��ϡ���� D.Na2CO3��ϡ����
����ͼ3��ƿ�м����ӷ����ʣ���ƾ�����ˮ���м�����ˮ���ټ������е��������������Ҳ��������Ȫ��ˮ���к��������ʿ�����___________��
A.Ũ���� B.ʳ�� C.��ʯ�� D.����
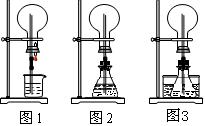
��2�������г�����������Ȫ����ɽ������ԭ��������___________���ӡ�ͼ1����ͼ2����ѡ��װ�õ�ԭ�����ơ�
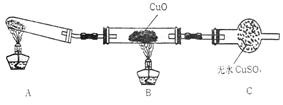
����С����ijͬѧ�����������ʾ��ʵ��
װ�ã��гּ�β������װ��δ��������̽������
�Ļ�ԭ�ԣ�
|
|
|
��2�����øĽ����װ�ý���ʵ�飬�۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4����ɫ��ͬʱ����һ������Ⱦ�����塣д��������CuO��Ӧ�Ļ�ѧ����ʽ ��

(15��)ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���
������ȡ��������Ȫ�����̽��
1.д��ʵ����ȡ�����Ļ�ѧ����ʽ ��
2.�ռ������ķ����� ��
3.��Ȫ��һ�ֳ�������Ȼ����
��1��ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�á���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ͼ3��ʾ��װ�á�
����ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����__________��
A.Cu��ϡ���� B.NaHCO3��NaOH��Һ C.MnO2��ϡ���� D.Na2CO3��ϡ����
����ͼ3��ƿ�м����ӷ����ʣ���ƾ�����ˮ���м�����ˮ���ټ������е��������������Ҳ��������Ȫ��ˮ���к��������ʿ�����___________��
A.Ũ���� B.ʳ�� C.��ʯ�� D.����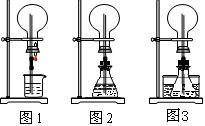
��2�������г�����������Ȫ����ɽ������ԭ��������___________���ӡ�ͼ1����ͼ2����ѡ��װ�õ�ԭ�����ơ�
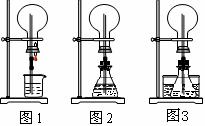
����С����ijͬѧ�����������ʾ��ʵ��
װ�ã��гּ�β������װ��δ��������̽������
�Ļ�ԭ�ԣ�
|
|
|



 ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���
ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���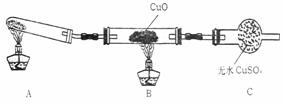 ����С����ijͬѧ�����������ʾ��ʵ��
����С����ijͬѧ�����������ʾ��ʵ��