��Ŀ����
���з�Ӧ�����ӷ���ʽ��ȷ����
| A�������ʵ����� Ba (OH )2�� NH4HSO4��ϡ��Һ��Ӧ Ba2+ + 2OHһ��2H+ + SO42����BaSO4����2H2O |
| B��������ͭ��Һ�м�������������������Һ Ba2+ʮSO42����BaSO4�� |
| C����̼��������Һ�е������ʯ��ˮ HCO3����Ca2+ʮOH����CaCO3��+H2O |
| D�����Ȼ�����Һ�м�������İ�ˮ A13++ 4NH3��H2O��Al(OH)3����4NH4+ +2H2O |
C
A ѡ����Ӧ�� NH3��H2O ���ɣ� B ѡ����©�� Cu2+��OHһ�ķ�Ӧ�� D ���а�ˮ����������ܽ� Al (OH)3������

��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�����Ŀ
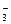 ��2OH��===CaCO3����2H2O��CO
��2OH��===CaCO3����2H2O��CO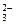
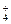 ��HCO
��HCO HSO
HSO ϡ��Һ���պó�����ȫ��
ϡ��Һ���պó�����ȫ��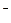 ��NH
��NH ��H
��H ��SO
��SO ====BaSO
====BaSO ��H
��H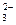 ��CO
��CO