��Ŀ����
���û�ѧ��Ӧԭ���о���������Ĵ�������Ӧ����Ҫ���壮
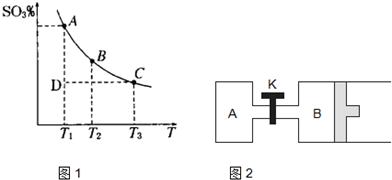
��1�����������У�SO2����������SO3��2SO2��g��+O2��g��?2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ1ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
��2SO2��g��+O2��g��?2SO3��g���ġ�H______0���������������
���ں��¡���ѹ������������ƽ����ϵ��ͨ�뺤����ƽ��______�ƶ�������淽������������������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1______K2��
��Ӧ���е�״̬Dʱ��v������______v���棩�����������������=������
��2����ͼ2��ʾ�����ر�Kʱ����A�г���2molSO2��1molO2����B�г���4molSO2��2molO2����ʼʱ��V��A��=V��B��=aL������ͬ�¶Ⱥ��д������ڵ������£��������з������з�Ӧ��2SO2��g��+O2��g��?2SO3��g���ﵽƽ�⣨��ʱ��V��B��=0.7aL����ش�
��B��X��ת���ʦ���SO2��BΪ______��
��A��SO2��B��O2�����ʵ����Ƚϣ�n��SO2��A______n��O2��B��ѡ�����������������=������
�۴�K����һ��ʱ�����´ﵽƽ�⣨ͼ 2��ʱ��B�����Ϊ______���ú�a�Ĵ���ʽ��ʾ����ͨ��������������Բ��ƣ���

��1�����������У�SO2����������SO3��2SO2��g��+O2��g��?2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ1ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
��2SO2��g��+O2��g��?2SO3��g���ġ�H______0���������������
���ں��¡���ѹ������������ƽ����ϵ��ͨ�뺤����ƽ��______�ƶ�������淽������������������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1______K2��
��Ӧ���е�״̬Dʱ��v������______v���棩�����������������=������
��2����ͼ2��ʾ�����ر�Kʱ����A�г���2molSO2��1molO2����B�г���4molSO2��2molO2����ʼʱ��V��A��=V��B��=aL������ͬ�¶Ⱥ��д������ڵ������£��������з������з�Ӧ��2SO2��g��+O2��g��?2SO3��g���ﵽƽ�⣨��ʱ��V��B��=0.7aL����ش�
��B��X��ת���ʦ���SO2��BΪ______��
��A��SO2��B��O2�����ʵ����Ƚϣ�n��SO2��A______n��O2��B��ѡ�����������������=������
�۴�K����һ��ʱ�����´ﵽƽ�⣨ͼ 2��ʱ��B�����Ϊ______���ú�a�Ĵ���ʽ��ʾ����ͨ��������������Բ��ƣ���
��1������ͼ��֪���¶�Խ�ߣ������ϵ��SO3�İٷֺ���ԽС��˵�������¶�ƽ�����淴Ӧ���У��������ƶ��������¶������ȷ�Ӧ�����ƶ������÷�Ӧ����ӦΪ���ȷ�Ӧ��
���¡���ѹ������������ƽ����ϵ��ͨ�뺤�������Ӧ����Ӧ��������ֵ�Ũ�Ƚ��ͣ���ЧΪ����ѹǿ��ѹǿ����ƽ��������������ƶ��������淽���ƶ���
�ʴ�Ϊ���������淽��
���¶����ߣ�ƽ�������ȷ����ƶ��������淴Ӧ�ƶ���Kֵ��С��K1��K2������D״̬δ��ƽ�⣬�����ϵ��SO3�İٷֺ���С��ƽ��ʱ�ģ���Ӧ������Ӧ���У�����v����v����
�ʴ�Ϊ����������
��2������B�ж��������ת����Ϊx��
���ݷ�Ӧ����ʽ��2SO2��g��+O2��g��?2SO3��g��
��Ӧǰ��mol��4 2 0
ת����mol�� 4x 2x 4x
ƽ�⣨mol�� 4-4x2-2x 4x
�ﵽƽ��ʱ��V��B��=0.7a L�����º�ѹ�����£����������֮�ȵ��ڻ����������ʵ���֮�ȣ�����4mol+2mol������4-4x+2-2x+4x��mol=a��0.7a��
��ã�x=0.9��
�ʴ�Ϊ��0.9��
��B�ں��º�ѹ�£�����4mol SO2��2mol O2��Ӧ�����2mol SO2��1mol O2��Ӧ��Ч������n��O2��=0.5n��SO2����
������װ��C����װ��C����2mol SO2��1mol O2��Ӧ��ѹǿ��B��ѹ�Һ��º�ѹ�����£��ﵽƽ��ʱ��BΪ��Чƽ�⣬��һ������n��SO2��B=2n��SO2��C=2n��O2��B��
����A�ݻ��㶨����Ӧ��ϵ��ѹǿС��C��ѹǿ���൱�ڼ�С��ѹǿ��ƽ�����������ƶ���A�ж�������ת����С��C�ģ���Ӧ��ﵽƽ��ʱn��SO2��A��n��SO2��C������n��SO2��C=n��O2��B������n��SO2��A��n��SO2��C=n��O2��B����n��SO2��A��n��O2��B��
�ʴ�Ϊ������
�۴�K����һ��ʱ�����´ﵽƽ�⣨ͼ 2��ʱ����B��ӦΪ��Чƽ�⣬�൱�ڼ�����6mol���������3mol������ͬ��ͬѹ�£��������ʵ�������������ȣ��ﵽƽ��ʱ�����Ϊ��0.7a��
=1.05a������B�����Ϊ1.05a-a=0.05a��
�ʴ�Ϊ��0.05a��
���¡���ѹ������������ƽ����ϵ��ͨ�뺤�������Ӧ����Ӧ��������ֵ�Ũ�Ƚ��ͣ���ЧΪ����ѹǿ��ѹǿ����ƽ��������������ƶ��������淽���ƶ���
�ʴ�Ϊ���������淽��
���¶����ߣ�ƽ�������ȷ����ƶ��������淴Ӧ�ƶ���Kֵ��С��K1��K2������D״̬δ��ƽ�⣬�����ϵ��SO3�İٷֺ���С��ƽ��ʱ�ģ���Ӧ������Ӧ���У�����v����v����
�ʴ�Ϊ����������
��2������B�ж��������ת����Ϊx��
���ݷ�Ӧ����ʽ��2SO2��g��+O2��g��?2SO3��g��
��Ӧǰ��mol��4 2 0
ת����mol�� 4x 2x 4x
ƽ�⣨mol�� 4-4x2-2x 4x
�ﵽƽ��ʱ��V��B��=0.7a L�����º�ѹ�����£����������֮�ȵ��ڻ����������ʵ���֮�ȣ�����4mol+2mol������4-4x+2-2x+4x��mol=a��0.7a��
��ã�x=0.9��
�ʴ�Ϊ��0.9��
��B�ں��º�ѹ�£�����4mol SO2��2mol O2��Ӧ�����2mol SO2��1mol O2��Ӧ��Ч������n��O2��=0.5n��SO2����
������װ��C����װ��C����2mol SO2��1mol O2��Ӧ��ѹǿ��B��ѹ�Һ��º�ѹ�����£��ﵽƽ��ʱ��BΪ��Чƽ�⣬��һ������n��SO2��B=2n��SO2��C=2n��O2��B��
����A�ݻ��㶨����Ӧ��ϵ��ѹǿС��C��ѹǿ���൱�ڼ�С��ѹǿ��ƽ�����������ƶ���A�ж�������ת����С��C�ģ���Ӧ��ﵽƽ��ʱn��SO2��A��n��SO2��C������n��SO2��C=n��O2��B������n��SO2��A��n��SO2��C=n��O2��B����n��SO2��A��n��O2��B��
�ʴ�Ϊ������
�۴�K����һ��ʱ�����´ﵽƽ�⣨ͼ 2��ʱ����B��ӦΪ��Чƽ�⣬�൱�ڼ�����6mol���������3mol������ͬ��ͬѹ�£��������ʵ�������������ȣ��ﵽƽ��ʱ�����Ϊ��0.7a��
| 9mol |
| 6mol |
�ʴ�Ϊ��0.05a��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ