��Ŀ����
�̲��и����ĸ����ʵĵ�һ�����ܵ��������£�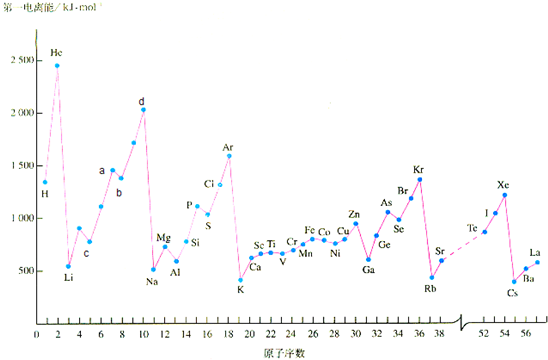
��ش��������⣺
��1��a�������Ԫ����
��2��d�������Ԫ�صĵ�һ�����ܴ��ڷ嶥ԭ���ǣ�
��3����ͼ��Ҳ�����˹���Ԫ�صĵ�һ���������ݣ����Ƕ��������ӵ��Ų��йأ���Cu����Χ�����Ų�ʽΪ��
��4��Mn Ԫ�ص���Χ�����Ų�ʽΪ3d54s2��FeԪ�ص���Χ�����Ų�ʽΪ3d64s2�������Mn�ĵ��������ܱ�Fe�ĵ��������ܴ��ԭ���ǣ�
��������1������a���λ���жϣ���VA��Ԫ��������nPΪ����״̬���ȶ���
��2��dΪNeԪ�أ��������8�����ȶ��ṹ�����ݼ۲���Ӷ����жϣ�
��3��Cu����Χ����Ϊȫ���Ͱ����Ų���
��4��ԭ�ӹ�����ڰ�����ȫ��ʱ���������ȶ���
��2��dΪNeԪ�أ��������8�����ȶ��ṹ�����ݼ۲���Ӷ����жϣ�
��3��Cu����Χ����Ϊȫ���Ͱ����Ų���
��4��ԭ�ӹ�����ڰ�����ȫ��ʱ���������ȶ���
����⣺��1������a���λ�ÿ�֪a�������Ԫ����N��NԪ��λ�ڵ�VA��Ԫ��������2PΪ����״̬���ȶ����ʴ�Ϊ��N��N��2p����������ʧȥһ�����ӽ��ѣ�
��2��dΪNeԪ�أ��������8�����ȶ��ṹ��ϡ�������е�Xe���γ�������XeO3����֪���ԭ������1�Թ¶Ե��ӣ�����Xe�ļ۲���Ӷ���Ϊ4����1�Թ¶Ե��ӣ������������Σ��ӻ�����Ϊsp3���ʴ�Ϊ��������Ѵﵽ8�����ȶ��ṹ��������sp3��
��3��Cu����Χ����Ϊȫ���Ͱ����Ų�������Cu����Χ�����Ų�ʽΪ��3d104s1���ʴ�Ϊ��3d104s1��
��4��MnԪ��Ϊ25��Ԫ�أ���������Ų�ʽΪ[Ar]3d54s2�����Լ۲�����Ų�ʽΪ3d54s2��
��Mn2+ת��ΪMn3+ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬תΪ���ȶ���3d4״̬��Ҫ�������϶ࣻ��Fe2+��Fe3+ʱ��3d�ܼ��ɲ��ȶ���3d6���ȶ���3d5�����״̬����Ҫ���������Ҫ�٣�
�ʴ�Ϊ����Mn2+ת��ΪMn3+ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬תΪ���ȶ���3d4״̬��Ҫ�������϶ࣻ��Fe2+��Fe3+ʱ��3d�ܼ��ɲ��ȶ���3d6���ȶ���3d5�����״̬����Ҫ���������Ҫ�٣�
��2��dΪNeԪ�أ��������8�����ȶ��ṹ��ϡ�������е�Xe���γ�������XeO3����֪���ԭ������1�Թ¶Ե��ӣ�����Xe�ļ۲���Ӷ���Ϊ4����1�Թ¶Ե��ӣ������������Σ��ӻ�����Ϊsp3���ʴ�Ϊ��������Ѵﵽ8�����ȶ��ṹ��������sp3��
��3��Cu����Χ����Ϊȫ���Ͱ����Ų�������Cu����Χ�����Ų�ʽΪ��3d104s1���ʴ�Ϊ��3d104s1��
��4��MnԪ��Ϊ25��Ԫ�أ���������Ų�ʽΪ[Ar]3d54s2�����Լ۲�����Ų�ʽΪ3d54s2��
��Mn2+ת��ΪMn3+ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬תΪ���ȶ���3d4״̬��Ҫ�������϶ࣻ��Fe2+��Fe3+ʱ��3d�ܼ��ɲ��ȶ���3d6���ȶ���3d5�����״̬����Ҫ���������Ҫ�٣�
�ʴ�Ϊ����Mn2+ת��ΪMn3+ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬תΪ���ȶ���3d4״̬��Ҫ�������϶ࣻ��Fe2+��Fe3+ʱ��3d�ܼ��ɲ��ȶ���3d6���ȶ���3d5�����״̬����Ҫ���������Ҫ�٣�
���������⿼������Ų�ʽ�������ܡ��ӻ������ӵĿռ乹�͵ȣ��漰֪ʶ��϶࣬�Ѷ��еȣ��Ƕ�֪ʶ�������ۺϿ��飮

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ