��Ŀ����
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ���ĸ���������H2�ķ�Ӧ����Zn+�����Na+ˮ ��Al+NaOH��Һ��Na+��ˮ�Ҵ���Ϊ��ȼ�����ĸ���Ӧ���ɵ�H2���������������װ��ͼ��

��ش��������⣺
��д��Na��H2O��Ӧ�Ļ�ѧ����ʽ ��
���ڵ�ȼH2֮ǰ�����Ƚ��� ��������
��
��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ���٢ۢ�ʵ���óɹ�����ȴ
ʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na��
����̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ
�յ�ԭ���� ��
��ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97g/mL��0.88g/mL��1.00g/mL�����ݴ˶�ʵ������˸Ľ���
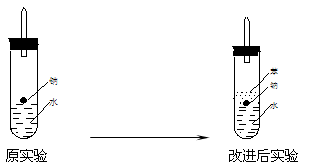
�ڸĽ����ʵ����H2���������ʼ�����ԭ����
��

��ش��������⣺
��д��Na��H2O��Ӧ�Ļ�ѧ����ʽ ��
���ڵ�ȼH2֮ǰ�����Ƚ��� ��������
��
��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ���٢ۢ�ʵ���óɹ�����ȴ
ʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na��
����̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ
�յ�ԭ���� ��
��ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97g/mL��0.88g/mL��1.00g/mL�����ݴ˶�ʵ������˸Ľ���

�ڸĽ����ʵ����H2���������ʼ�����ԭ����
��
��2Na+2H2O��2NaOH+H2��
���鴿���������������ռ�һ�Թ���������Ĵָ��ס���ƽ����棬�ƿ�Ĵָ������������ġ��ˡ����������H2������
�ǽ϶������ˮ��Ӧ�ų��������ȣ�ʹ�Թ���H2��O2�Ļ������ȼ����ը��
���Ʊ�ˮ�ᣬ�ȱ��أ����ڱ�ˮ���紦������H2O��Ӧ������H2ʹ�Ƹ�������ˮ�棬��Ӧֹͣ�����Ʊ����H2�ݳ������ֻ��䣬��ˮ��Ӧ����˷������Ϳɼ���Na��H2O��Ӧ�ٶȡ�
���鴿���������������ռ�һ�Թ���������Ĵָ��ס���ƽ����棬�ƿ�Ĵָ������������ġ��ˡ����������H2������
�ǽ϶������ˮ��Ӧ�ų��������ȣ�ʹ�Թ���H2��O2�Ļ������ȼ����ը��
���Ʊ�ˮ�ᣬ�ȱ��أ����ڱ�ˮ���紦������H2O��Ӧ������H2ʹ�Ƹ�������ˮ�棬��Ӧֹͣ�����Ʊ����H2�ݳ������ֻ��䣬��ˮ��Ӧ����˷������Ϳɼ���Na��H2O��Ӧ�ٶȡ�
�����ǻ��õĽ�������H2O��Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O��2NaOH+H2����
��2�������ǿ�ȼ�����壬�ڵ�ȼH2֮ǰ�����Ƚ����鴿��һ����ñ����������������������ռ�һ�Թ���������Ĵָ��ס���ƽ����棬�ƿ�Ĵָ������������ġ��ˡ����������H2������
��3���������ǻ��õĽ������϶������ˮ��Ӧ�ų��������ȣ�ʹ�Թ���H2��O2�Ļ������ȼ����ը��
��4���Ʊ�ˮ�ᣬ���ȱ��أ������ƽ����ڱ���ˮ�Ľ��紦������H2O��Ӧ������H2ʹ�Ƹ�������ˮ�棬��Ӧֹͣ�����Ʊ����H2�ݳ������ֻ��䣬��ˮ��Ӧ����˷������Ϳɼ���Na��H2O��Ӧ�ٶȡ�
��2�������ǿ�ȼ�����壬�ڵ�ȼH2֮ǰ�����Ƚ����鴿��һ����ñ����������������������ռ�һ�Թ���������Ĵָ��ס���ƽ����棬�ƿ�Ĵָ������������ġ��ˡ����������H2������
��3���������ǻ��õĽ������϶������ˮ��Ӧ�ų��������ȣ�ʹ�Թ���H2��O2�Ļ������ȼ����ը��
��4���Ʊ�ˮ�ᣬ���ȱ��أ������ƽ����ڱ���ˮ�Ľ��紦������H2O��Ӧ������H2ʹ�Ƹ�������ˮ�棬��Ӧֹͣ�����Ʊ����H2�ݳ������ֻ��䣬��ˮ��Ӧ����˷������Ϳɼ���Na��H2O��Ӧ�ٶȡ�

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д� �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ

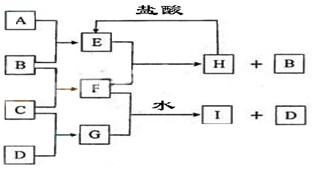
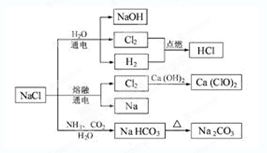
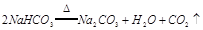
 2Na+Cl2��
2Na+Cl2��
 II. ��0.6 g̿����3.9 g Na2O2���Ȼ�ϣ�װ���Թܣ��ڿ����Թܿڴ�����һ��ʪ���
II. ��0.6 g̿����3.9 g Na2O2���Ȼ�ϣ�װ���Թܣ��ڿ����Թܿڴ�����һ��ʪ��� Na2CO3+X����XΪ (�ѧʽ),�������ʵ��֤������X�Ĵ���,��Ҫд����������������ͽ��ۣ�
Na2CO3+X����XΪ (�ѧʽ),�������ʵ��֤������X�Ĵ���,��Ҫд����������������ͽ��ۣ�