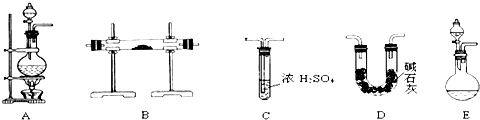
��Ŀ����
ʵ�����ø��������Cl2�����ڵ�Sn��Ӧ��SnCl4��Sn+2Cl2
��1��װ��A�еķ�Ӧԭ��������Ϊ��MnO2��ŨH2SO4��ʳ�η�Ӧ������HCl������Cl2����������������NaHSO4��MnSO4��װ��A�з�Ӧ�Ļ�ѧ����ʽ______________________
________________________________��
��2��Ϊʹʵ��ɹ���A��B����Ҫ���ʵ���װ�ã��뽫�����ڿ��ڣ���ע������ʢ�ŵ�ҩƷ��
��3������ʵ��ʱ��Ӧ�ȵ�ȼ����д��ĸ����ͬ��________���ľƾ��ƣ�����Ӧ����SnCl4ʱ��ӦϨ��________���ľƾ��ƣ�������________________________��
��4��װ��C��������________________________________��
��5�����д�ʵ�飬Dװ�ú�Ӧ�����ӵ�װ�ü������ǣ��������ҵ�˳��д���������Ƽ����е�ҩƷ��___________________________________________________________________
___________________________________________________________________________________________________________________________________________________________________________________________________________________________________________��
������
| ��1��2NaCl+3H2SO4+MnO2 ��2��
��3��B B ������Ӧ�ų�����ά��Sn���ۻ� ��4��ʹSnCl4�������� ��5�����Ӹ���ܣ�װ�м�ʯ�ң���ֹˮ����������ƿʹSnCl4����ˮ�⡣����Cl2��β������װ�ã�װ��NaOH��Һ������Cl2�������������������Ӹ���ܣ�װ�м�ʯ�ң���ֹ������ˮ����������ƿ��ʹSnCl4����ˮ�⣻����Cl2��������������
|


 ��1����μ��Dװ�õ������ԣ�
��1����μ��Dװ�õ������ԣ�