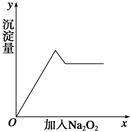
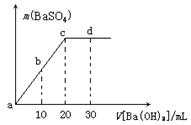
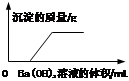��Ŀ����
(20��)��1����������״̬�����ʢٸɱ� ��NaCl���� �۰�ˮ �ܴ��� �ݾƾ�ˮ��Һ ��ͭ �����ڵ�KOH �����ǣ��������ڵ���ʵ��� �����ڷǵ���ʵ��� ���ܵ������ �� �����ܵ���ĵ���ʵĵ��뷽��ʽ�� ��
��2�������������ӵ�������Һ ��Ag+ ��Mg2+ ��Fe2+ ��Al3+ ��Fe3+ �Իش��������⣺
�ټ��ܱ��������ܱ���ԭ��������____________�������ӷ��ţ���ͬ��
������������Cl�C���ڵ�������___________
�ۼ������NaOH��Һ��������_____________
�ܼ�������Һ�������ص���_________,��Һ���������___________
����KSCN��Һ�ʺ�ɫ����_________________________
��3����Ҫ��д�����з���ʽ��
�� �������ĵ��뷽��ʽ�� ��
�� С�մ�����θ���������ӷ���ʽ�� ��
�� ��FeSO4��Һ�м���NaOH��Һ,������
�йػ�ѧ����ʽΪ ��
��2�������������ӵ�������Һ ��Ag+ ��Mg2+ ��Fe2+ ��Al3+ ��Fe3+ �Իش��������⣺
�ټ��ܱ��������ܱ���ԭ��������____________�������ӷ��ţ���ͬ��
������������Cl�C���ڵ�������___________
�ۼ������NaOH��Һ��������_____________
�ܼ�������Һ�������ص���_________,��Һ���������___________
����KSCN��Һ�ʺ�ɫ����_________________________
��3����Ҫ��д�����з���ʽ��
�� �������ĵ��뷽��ʽ�� ��
�� С�մ�����θ���������ӷ���ʽ�� ��
�� ��FeSO4��Һ�м���NaOH��Һ,������
�йػ�ѧ����ʽΪ ��
��1���ڢܢߣ� �٢࣬ �ۢޢߣ�KOH=K++OH-
��2����Fe2+ �� Ag+ ��Al3+ �� Fe3+ ; Ag+ ��Fe3+������ÿ�ո�1��,��10�֣�
 (3)�� CH3COOH CH3COO- +H+
(3)�� CH3COOH CH3COO- +H+��HCO3- + H+=CO2��+H2O
�����а�ɫ�������ɣ�Ѹ��ת��Ϊ����ɫ�����ձ�Ϊ���ɫ��
FeSO4+2NaOH=Fe(OH)2��+Na2SO4
4Fe(OH)2+O2 +2H2O="=4" Fe(OH)3��ÿ�ո�2�֣���10�֣�
�����������1���������ָ��ˮ��Һ������״̬�¿��Ե���Ļ�����ǵ��������ˮ��Һ������״̬�¶����ܵ���Ļ�����ǵ�����Тٸɱ�������̬������̼�������ǣ�������� ��NaCl���� �ܴ��� �����ڵ�KOH �����۰�ˮ�ݾƾ�ˮ��Һ�ǻ�����ͭ�ǵ��ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʡ�Ӧע����Ե���ĵ���ʣ���ָ���������״̬�¿��Ե���ĵ���ʣ�����������ֻ�����ڵ�KOH��
��2����Fe2+���Ա�������Fe3+Ҳ���Ա���ԭ��Fe���ʢ� Ag+����Cl�C��Ӧ���ɳ�����Al3+ ��Һ�м������NaOH��Һ�������ɳ��������ܽ�����ۿ���Fe3+��Ag+ ��Ӧ��ǰ����Һ�������ӣ�����Ag+ ���û���Fe2+���������ʣ���Һ������С����Fe3+��KSCN��Һ�ʺ�ɫ
(3)�� CH3COOH���뷽��ʽ����д��ע�������ţ�
��NaHCO3�����ᷴӦ�����ӷ���ʽ����д��
�����а�ɫ�������ɣ�Ѹ��ת��Ϊ����ɫ�����ձ�Ϊ���ɫ��
FeSO4+2NaOH=Fe(OH)2��+Na2SO4
4Fe(OH)2+O2 +2H2O="=4" Fe(OH)3
���������⿼�����ʺͷǵ���ʵĸ�����ӷ�Ӧ���������������ӷ���ʽ����д�����ݽϻ�����

��ϰ��ϵ�д�
�����Ŀ
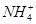 ��
��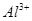 ��
��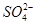 ����
����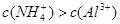
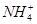 ��
��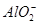 ��
��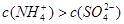
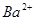 ��
��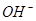 ����
����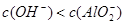
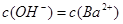
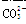 ��
��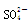 ��
��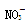 �������е������֡���������ʵ�飺
�������е������֡���������ʵ�飺