��Ŀ����
���б�ʾ��Һ����������Ӧ�����ӷ���ʽ��ȷ���� �� ��
A��NH4HCO3��Һ�����KOHŨ��Һ���ȣ�NH4++ OH��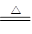 NH3��+ H2O NH3��+ H2O |
| B��Ca(HCO3)2��Һ�м�������NaOH��Һ�� Ca2��+2HCO3��+2OH����CaCO3��+CO32��+H2O |
| C��KI��Һ��H2SO4�ữ��H2O2��Һ��ϣ� 2I��+ H2O2 + 2H+ =2H2O + I2 |
| D�����Ȼ�������Һ�м��뱥�͵⻯����Һ��Ag+ +I����AgI �� |
C
A�У�©����HCO3-��OH���ķ�Ӧ��B�У�NaOH��Һ���٣���Ӧ���ˣ�ϵ��Ӧ����1��D�У��Ȼ����dz��������ܲ�

��ϰ��ϵ�д�
�����Ŀ
 Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O Na+ + OH�� + H2��
Na+ + OH�� + H2��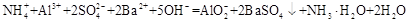
 2OH��+H2��+Cl2��
2OH��+H2��+Cl2��