��Ŀ����
ʳ�Ρ�̼���ƺ�̼�������������г��������Ρ���ش��������⡣
��1��̼�����Ƶ�ˮ��Һ��__________�ԡ�����ᡱ��������С�������ȥ̼���ƹ����л��е�����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ_________________��
��2����������̼���ƺ�̼�����Ʒֱ����������ᷴӦʱ���ɵ�CO2����ǰ��________���ߣ��>������<����=������
��3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl������Ϊ�ܽ⡢�ӹ���a���ӹ���NaOH���ӹ���b�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣�Լ�a��b�ֱ���________(����ţ�
A.Na2CO3 BaCl2 B.BaCl2 Na2CO3 C.BaCl2 Na2SO4
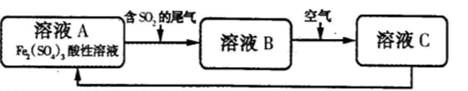
��4����ҵ���õ�ⱥ��ʳ��ˮ�ķ��������������ռ
��ij��������������й©�¼���������Ա����NaOH��Һ�γ�ҺĻ����Χ������й©���������䷴Ӧԭ��_________________________________�������ӷ���ʽ��ʾ����
�ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©������Ӧ����ʽ���£�
 �÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
�÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
��1��̼�����Ƶ�ˮ��Һ��__________�ԡ�����ᡱ��������С�������ȥ̼���ƹ����л��е�����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ_________________��
��2����������̼���ƺ�̼�����Ʒֱ����������ᷴӦʱ���ɵ�CO2����ǰ��________���ߣ��>������<����=������
��3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl������Ϊ�ܽ⡢�ӹ���a���ӹ���NaOH���ӹ���b�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣�Լ�a��b�ֱ���________(����ţ�
A.Na2CO3 BaCl2 B.BaCl2 Na2CO3 C.BaCl2 Na2SO4
��4����ҵ���õ�ⱥ��ʳ��ˮ�ķ��������������ռ
��ij��������������й©�¼���������Ա����NaOH��Һ�γ�ҺĻ����Χ������й©���������䷴Ӧԭ��_________________________________�������ӷ���ʽ��ʾ����
�ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©������Ӧ����ʽ���£�
 �÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
�÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________����1���� 2NaHCO3 Na2CO3 + CO2��+ H2O��2���� ��3��B��4����Cl2 + 2OH-=Cl- + ClO- + H2O�����ȣ�3:2��
Na2CO3 + CO2��+ H2O��2���� ��3��B��4����Cl2 + 2OH-=Cl- + ClO- + H2O�����ȣ�3:2��
 Na2CO3 + CO2��+ H2O��2���� ��3��B��4����Cl2 + 2OH-=Cl- + ClO- + H2O�����ȣ�3:2��
Na2CO3 + CO2��+ H2O��2���� ��3��B��4����Cl2 + 2OH-=Cl- + ClO- + H2O�����ȣ�3:2�������������1��̼�����Ƶ�ˮ��Һ�Լ��ԣ�̼���������ȷֽ�����̼���ơ�������̼��ˮ��̼���������ѷֽ⣬��ȥ̼���ƹ����л��е�����̼�����ƿ��ü��ȷ�����Ӧ�Ļ�ѧ����ʽΪ2NaHCO3
 Na2CO3 + CO2��+ H2O����2��̼���Ƶ���Է�������Ϊ106��̼�����Ƶ���Է�������Ϊ84����������̼���ƺ�̼�����ƣ�̼���Ƶ����ʵ���С��̼�����Ƶ����ʵ���������̼�غ��֪���ֱ����������ᷴӦʱ���ɵ�CO2����ǰ��С�ں��ߣ���3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl��Ϊ���������ӳ����������Լ�Ҫ�������������Լ����ں��������б����ȥ�������������Ƴ�ȥþ���ӣ����Ȼ�����ȥ���������̼���Ƴ�ȥ�����Ӽ������ı����ӣ�ʵ�鲽��Ϊ�ܽ⡢�ӹ���BaCl2���ӹ���NaOH���ӹ���Na2CO3�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣ѡB����4��������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ���ʽΪCl2 + 2OH-=Cl- + ClO- + H2O���ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©�����÷�Ӧ�У���Ԫ�صĻ��ϼ۽��ͣ�����ԭ���������������������е�Ԫ�صĻ��ϼ����ߣ�����������������ԭ�����μӵİ�����ֻ��2�����Ӱ������������÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ3:2��
Na2CO3 + CO2��+ H2O����2��̼���Ƶ���Է�������Ϊ106��̼�����Ƶ���Է�������Ϊ84����������̼���ƺ�̼�����ƣ�̼���Ƶ����ʵ���С��̼�����Ƶ����ʵ���������̼�غ��֪���ֱ����������ᷴӦʱ���ɵ�CO2����ǰ��С�ں��ߣ���3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl��Ϊ���������ӳ����������Լ�Ҫ�������������Լ����ں��������б����ȥ�������������Ƴ�ȥþ���ӣ����Ȼ�����ȥ���������̼���Ƴ�ȥ�����Ӽ������ı����ӣ�ʵ�鲽��Ϊ�ܽ⡢�ӹ���BaCl2���ӹ���NaOH���ӹ���Na2CO3�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣ѡB����4��������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ���ʽΪCl2 + 2OH-=Cl- + ClO- + H2O���ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©�����÷�Ӧ�У���Ԫ�صĻ��ϼ۽��ͣ�����ԭ���������������������е�Ԫ�صĻ��ϼ����ߣ�����������������ԭ�����μӵİ�����ֻ��2�����Ӱ������������÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ3:2��
��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д�
�����Ŀ

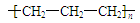
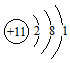
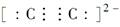

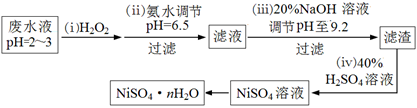
 HClO + HCl
HClO + HCl