��Ŀ����
��2013?����һģ�������£���ijһԪ��HA��NaOH��Һ�������ϣ�ʵ����Ϣ���£�
|
����������ʵ���֪�������Ũ�ȷ�Ӧ��Һ�ʼ��ԣ��ж�HA�����ᣬ����Һ�д��ڵ���ƽ�⣻
A�����ݻ����ҺPH=7˵����Һ�����ԣ����ݵ���غ�����жϣ�
B�������ж�HA��������ڵ���ƽ�⣻
C����Ӧ������NaA��Һ��A-����ˮ���Լ��ԣ�
D��������Һ�е���غ�����жϣ�
A�����ݻ����ҺPH=7˵����Һ�����ԣ����ݵ���غ�����жϣ�
B�������ж�HA��������ڵ���ƽ�⣻
C����Ӧ������NaA��Һ��A-����ˮ���Լ��ԣ�
D��������Һ�е���غ�����жϣ�
����⣺A����Һ��һ������c��OH-��+c��A-��=c��Na+��+c��H+���������ҺPH=7˵����Һ�����ԣ�c��OH-��=c��H+��������c��A-��=c��Na+����ԭ��ҺŨ��c1��0.2 mol?L-1����A��ȷ��
B��HA��������ڵ���ƽ�⣬HA�ĵ��뷽��ʽ��HA?H++A-����B��ȷ��
C����Ӧ������NaA��Һ��A-����ˮ���Լ��ԣ���Һ������Ũ�ȴ�Сc��Na+����c��A-����c��OH-����c��H+������C����
D������Һ��һ������c��OH-��+c��A-��=c��Na+��+c��H+���������ҺPH=7˵����Һ�����ԣ�c��OH-��=c��H+��������c��A-��=c��Na+����c��Na+����c��HA��+c��A-������D��ȷ��
��ѡC��
B��HA��������ڵ���ƽ�⣬HA�ĵ��뷽��ʽ��HA?H++A-����B��ȷ��
C����Ӧ������NaA��Һ��A-����ˮ���Լ��ԣ���Һ������Ũ�ȴ�Сc��Na+����c��A-����c��OH-����c��H+������C����
D������Һ��һ������c��OH-��+c��A-��=c��Na+��+c��H+���������ҺPH=7˵����Һ�����ԣ�c��OH-��=c��H+��������c��A-��=c��Na+����c��Na+����c��HA��+c��A-������D��ȷ��
��ѡC��
���������⿼����Ӧ����Һ����Եķ����жϣ�������ʷ�������Һ�е���غ��Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
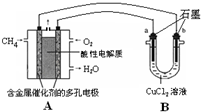 ��2013?����һģ����������Դ��ʹ�����ȼ�ϣ����Դﵽ�����Ч��������Ⱦ��Ŀ�ģ�
��2013?����һģ����������Դ��ʹ�����ȼ�ϣ����Դﵽ�����Ч��������Ⱦ��Ŀ�ģ�