��Ŀ����
��10�֣���������������ֹʹ�������á��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3��S��CH3��������������NO2��Ӧ��ȡ�������� ( )���йط�Ӧ���£�
)���йط�Ӧ���£�
��Ӧ�� 2CH3OH(l)��H2S(g)��(CH3)2S(l) ��2H2O(l) ��H���DakJ��mol-1
��Ӧ�� (CH3)2S(l)��NO2(g)��(CH3)2SO(l)��NO(g) ��H����bkJ����mol-1
��Ӧ�� 2NO(g)��O2(g)��2NO2(g) ��H����ckJ��mol-1
��1��д���ü�����ֱ�Ӻ�������Ӧ��ȡ�����������Ȼ�ѧ��Ӧ����ʽ
___________________________________________________��
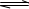 ��2����˵����Ӧ2CH3OH(l)��H2S(g)
(CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________
��
��2����˵����Ӧ2CH3OH(l)��H2S(g)
(CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________
��
A. v(CH3OH) = 2v(H2S)
B. ���������У���ϵ��ѹǿ���ٸı�
C. ���������У���ϵ��������ܶȲ��ٸı�
D. ���������У������Ħ���������ٸı�
��3����Ӧ����һ�������¿ɴﵽƽ�⣬��������¸÷�Ӧƽ�ⳣ������ʽK= ____________________��
��4��N2O5��һ��������ɫ�����������Ʊ��������������֡�
����һ��4NO2(g)��O2(g) ��2N2O5(g)�� ��H����56.76 KJ��mol-1 [��Դ:ѧ#��#��Z#X#X#K]
�����£��÷�Ӧ�������Է����У�������Ӧ�ġ�S __________ 0�����������������
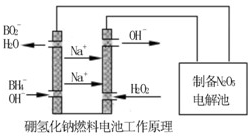
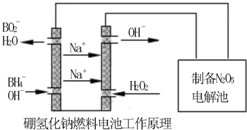
�������������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5������ԭ������ͼ��

���⻯��ȼ�ϵ�ص�������Ӧʽ________________________��
��10�֣�
��1��2(CH3)2S(l) ��O2(g)��2 (CH3)2SO(l) ��H������2b+c��kJ��mol-1��2�֣�
��2��B C ��2�֣���1�֣�
��3��K=c2(NO2)/c2(NO) c(O2) ��2�֣�
��4������2�֣��� H2O2��2e-��2OH����2�֣�
��������

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д���10�֣���������������ֹʹ�������á��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3��S��CH3��������������NO2��Ӧ��ȡ�������� (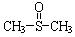 )���йط�Ӧ���£�
)���йط�Ӧ���£�
��Ӧ�� 2CH3OH(l)��H2S(g)��(CH3)2S(l) ��2H2O(l) ��H���DakJ��mol-1
��Ӧ�� (CH3)2S(l)��NO2(g)��(CH3)2SO(l)��NO(g) ��H����bkJ����mol-1
��Ӧ�� 2NO(g)��O2(g)��2NO2(g) ��H����ckJ��mol-1
��1��д���ü�����ֱ�Ӻ�������Ӧ��ȡ�����������Ȼ�ѧ��Ӧ����ʽ
___________________________________________________�� ��2����˵����Ӧ2CH3OH(l)��H2S(g) (CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________ ��
��2����˵����Ӧ2CH3OH(l)��H2S(g) (CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________ ��
| A��v(CH3OH) =" 2v(H2S)" |
| B�����������У���ϵ��ѹǿ���ٸı� |
| C�����������У���ϵ��������ܶȲ��ٸı� |
D�����������У������Ħ�������� �ٸı� �ٸı� |
��4��N2O5��һ��������ɫ�����������Ʊ��������������֡�
����һ��4NO2(g)��O2(g) ��2N2O5(g)
 ����H����56.76 KJ��mol-1
����H����56.76 KJ��mol-1 �����£��÷�Ӧ�������Է����У�������Ӧ�ġ�S __________ 0�����������������
�������������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5������ԭ������ͼ��
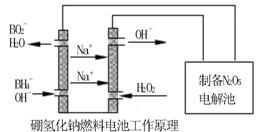
���⻯��ȼ�ϵ�ص�������Ӧʽ________________________��
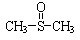 �����йط�Ӧ���£�
�����йط�Ӧ���£�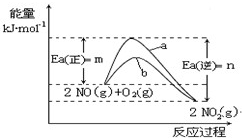
 ��������
��������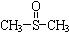 ������ֹʹ�������ã��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3-S-CH3��������������NO2��Ӧ��ȡ����������
������ֹʹ�������ã��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3-S-CH3��������������NO2��Ӧ��ȡ����������