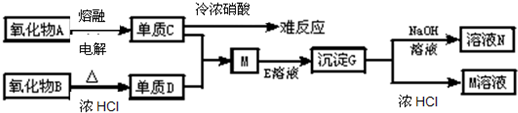
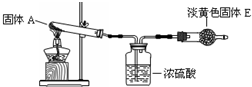
��Ŀ����
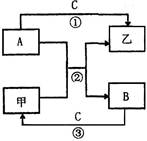
���н�������A��B��H��I������ס��ҡ���������C��D��E��F��G������H�ǵؿ��к������Ľ���������֮���ܷ������·�Ӧ��ͼ����Щ��Ӧ�IJ���ͷ�Ӧ������û��ȫ���������

�����������Ϣ�ش��������⣺
��1��д���������ʵĻ�ѧʽ��B______����______��
��2��д����Ӧ�ٵ����ӷ���ʽ��______��
��3��д����Ӧ�ܵ����ӷ���ʽ��______��
��4��ʵ���Ҽ�������F�������ӵij����Լ�Ϊ______��
��5��H��C��Ӧ�Ļ�ѧ����ʽ��______��

�����������Ϣ�ش��������⣺
��1��д���������ʵĻ�ѧʽ��B______����______��
��2��д����Ӧ�ٵ����ӷ���ʽ��______��
��3��д����Ӧ�ܵ����ӷ���ʽ��______��
��4��ʵ���Ҽ�������F�������ӵij����Լ�Ϊ______��
��5��H��C��Ӧ�Ļ�ѧ����ʽ��______��
����A����ɫ��ӦΪ��ɫ��ӦΪNa�����ΪH2��CΪNaOH��HӦΪAl������ɫ������ΪCl2����ΪHCl��DΪ���ᣬ���ɫ����GΪFe��OH��3��FΪFeCl3��EΪFeCl2��BΪFe���Ϻ�ɫ����IΪCu��
��1�������Ϸ�����֪BΪFe����ΪCl2���ʴ�Ϊ��Fe��Cl2��
��2����Ӧ��Ϊ�ƺ�ˮ�ķ�Ӧ�����ӷ���ʽΪ2Na+2H2O=2Na++2OH-+H2�����ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����
��3����Ӧ��ΪFeCl2�������ķ�Ӧ����Ӧ�����ӷ���ʽΪ2Fe2++Cl2=2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2=2Fe3++2Cl-��
��4��FΪFeCl3������KSCN���ɫ������KSCN���飬�ʴ�Ϊ��KSCN��
��5��HΪAl��CΪNaOH����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��1�������Ϸ�����֪BΪFe����ΪCl2���ʴ�Ϊ��Fe��Cl2��
��2����Ӧ��Ϊ�ƺ�ˮ�ķ�Ӧ�����ӷ���ʽΪ2Na+2H2O=2Na++2OH-+H2�����ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����
��3����Ӧ��ΪFeCl2�������ķ�Ӧ����Ӧ�����ӷ���ʽΪ2Fe2++Cl2=2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2=2Fe3++2Cl-��
��4��FΪFeCl3������KSCN���ɫ������KSCN���飬�ʴ�Ϊ��KSCN��
��5��HΪAl��CΪNaOH����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ