��Ŀ����
��16�֣��蹸���ɷ�ֹ��ҵ��ˮ��������������Ṹ���������з�Ӧ·�߿ɵõ�E��R�����蹸�������ַ�Ӧ������ȥ����
��1���蹸��E���Ʊ�
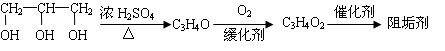
�� A����������Ҫ��Ӫ������________ˮ���Ƶã�����ࡱ������֬�������ʡ�����
�� B�����Ƶ�Cu(OH)2��Ӧ����D���仯ѧ����ʽΪ__________ ____��
____��
�� D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ______________��
��2���蹸��R���Ʊ�
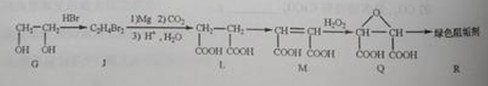
�� Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
��Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ�� __________________��
����L�Ʊ�M�ķ�Ӧ��������Ϊ��
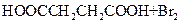
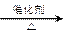
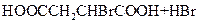 ��_____��_____���û�ѧ����ʽ��ʾ����
��_____��_____���û�ѧ����ʽ��ʾ����
��1 mol Q��ͬ���칹��T��̼����֧����������NaHCO3��Һ���ò���2 mol CO2��T�Ľṹ��ʽΪ__________��ֻдһ�֣���
��1���蹸��E���Ʊ�

�� A����������Ҫ��Ӫ������________ˮ���Ƶã�����ࡱ������֬�������ʡ�����
�� B�����Ƶ�Cu(OH)2��Ӧ����D���仯ѧ����ʽΪ__________
 ____��
____���� D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ______________��
��2���蹸��R���Ʊ�

��
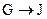 Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________����Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ�� __________________��
����L�Ʊ�M�ķ�Ӧ��������Ϊ��
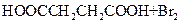
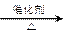
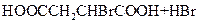 ��_____��_____���û�ѧ����ʽ��ʾ����
��_____��_____���û�ѧ����ʽ��ʾ������1 mol Q��ͬ���칹��T��̼����֧����������NaHCO3��Һ���ò���2 mol CO2��T�Ľṹ��ʽΪ__________��ֻдһ�֣���
��1������֬
��

��

(2) ��CH2BrCH2Br
��CO2
��HOOCCH2CHBrCOOH+3NaOH
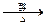 NaOOCCH=CHOONa+NaBr+3H2O
NaOOCCH=CHOONa+NaBr+3H2ONaOOCCH=CHCOONa+H2SO4��HOOCCH==CHCOOH+Na2SO.
��

��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
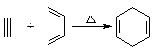 �����Ҫ�ϳ�
�����Ҫ�ϳ� �����õ�ԭ�Ͽ����� ��
�����õ�ԭ�Ͽ����� �� 
 F���������������У���ṹ��ʽ
F���������������У���ṹ��ʽ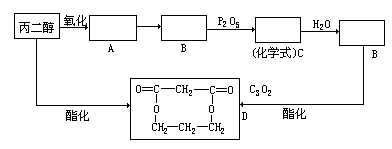


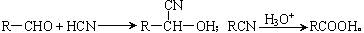
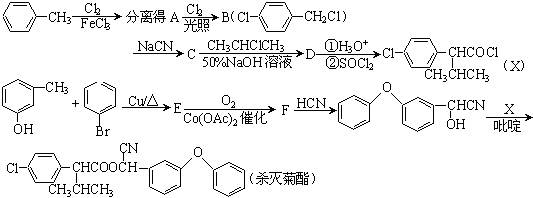

 ���ס��ҡ������DZ��ͻ������ҷ����ж�����26�����ӡ��ݴ��ƶϣ�
���ס��ҡ������DZ��ͻ������ҷ����ж�����26�����ӡ��ݴ��ƶϣ� ��Fԭ�Ӵ��棬���ò��������_______�ֽṹ��
��Fԭ�Ӵ��棬���ò��������_______�ֽṹ�� �� B������ӹ���Ϊ���ı���
�� B������ӹ���Ϊ���ı���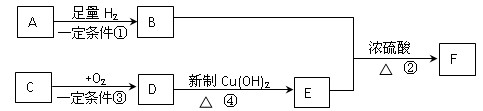
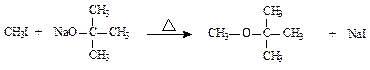
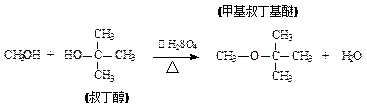
 ( )
( )