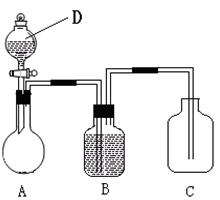
��Ŀ����
ʱ�ӷ�Ӧ����Ӧ�ṩ�����˿�����ʾʵ�飬Ҳ��һ����Ծ���о����ⶨ����I��Ũ�Ⱥ�С�ĵ⻯����Һʱ��������Ӧ���л�ѧ�Ŵ������ԭ��Һ�е����ӵ�Ũ�ȡ���Ҫ�������ǣ�
����������Һ�У����彫������I��������IO3���������������ȥ��
���ټ��������KI�������������£�ʹIO3����ȫת����I2��
�۽��������ɵĵ���ȫ��ȡ�����½��仹ԭ��I��������ʽΪ��N2H4+2I2=4I��+N2+4H+
�ܽ����ɵ�I����ȡ��ˮ����âٷ�������
�ݽ��ܵõ�����Һ����������KI��Һ�����������ữ��
���ݷ�Ӧ�����Һ�Ե�����ָʾ������Na2S2O3����Һ�ζ�������ʽΪ��
2Na2S2O3+I2 Na2S4O6+2NaI���������Ŵ����Һ��I��Ũ�ȷŴ�Ϊԭ��Һ��I��Ũ�ȵģ���ǰ����Һ�����ȣ�
Na2S4O6+2NaI���������Ŵ����Һ��I��Ũ�ȷŴ�Ϊԭ��Һ��I��Ũ�ȵģ���ǰ����Һ�����ȣ�
����������Һ�У����彫������I��������IO3���������������ȥ��
���ټ��������KI�������������£�ʹIO3����ȫת����I2��
�۽��������ɵĵ���ȫ��ȡ�����½��仹ԭ��I��������ʽΪ��N2H4+2I2=4I��+N2+4H+
�ܽ����ɵ�I����ȡ��ˮ����âٷ�������
�ݽ��ܵõ�����Һ����������KI��Һ�����������ữ��
���ݷ�Ӧ�����Һ�Ե�����ָʾ������Na2S2O3����Һ�ζ�������ʽΪ��
2Na2S2O3+I2
 Na2S4O6+2NaI���������Ŵ����Һ��I��Ũ�ȷŴ�Ϊԭ��Һ��I��Ũ�ȵģ���ǰ����Һ�����ȣ�
Na2S4O6+2NaI���������Ŵ����Һ��I��Ũ�ȷŴ�Ϊԭ��Һ��I��Ũ�ȵģ���ǰ����Һ�����ȣ�| A��2�� | B��4�� | C��36�� | D��6�� |
C
�ٵ�һ����I��+3Br2+3H2O=6Br��+IO3��+6H����I��Ũ�Ȳ��䣻
�ڵڶ�����IO3��+5I��+6H��=3I2+3H2O��1��IO3������3��I2���ӵ�������Ӧ������I��Ũ�ȷŴ���6����
�۵�������N2H4+2I2=4I��+N2��+4H����1��I2����2��I����
�ܵ��IJ���I��+3Br2+3H2O=6Br��+IO3��+6H����I��Ũ�Ȳ��䣻
�ݵ��岽��IO3��+5I��+6H��=3I2+3H2O��1��IO3������3��I2���ӵ�������Ӧ������I��Ũ�ȷŴ���6����
��������2Na2S2O3+I2=Na2S4O6+2NaI�����ӷ���ʽΪ��2S2O32��+I2=S4O62��+2I����1��I2����2��I����
��˴����Ϸ���ʽ�ͷ����ɵù�ϵʽ��I��IO3����3I2��6I����6IO3����18I2��36S2SO32����36I����
1 3 6 6 18 36
�ܼƷŴ���Ϊ��6��6=36����
��ѡC��
�ڵڶ�����IO3��+5I��+6H��=3I2+3H2O��1��IO3������3��I2���ӵ�������Ӧ������I��Ũ�ȷŴ���6����
�۵�������N2H4+2I2=4I��+N2��+4H����1��I2����2��I����
�ܵ��IJ���I��+3Br2+3H2O=6Br��+IO3��+6H����I��Ũ�Ȳ��䣻
�ݵ��岽��IO3��+5I��+6H��=3I2+3H2O��1��IO3������3��I2���ӵ�������Ӧ������I��Ũ�ȷŴ���6����
��������2Na2S2O3+I2=Na2S4O6+2NaI�����ӷ���ʽΪ��2S2O32��+I2=S4O62��+2I����1��I2����2��I����
��˴����Ϸ���ʽ�ͷ����ɵù�ϵʽ��I��IO3����3I2��6I����6IO3����18I2��36S2SO32����36I����
1 3 6 6 18 36
�ܼƷŴ���Ϊ��6��6=36����
��ѡC��

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
 ����Ϊ98%���ܶ�Ϊ1.84g/�M3��Ũ����______ mL����С�������1λ��Ч���֣�
����Ϊ98%���ܶ�Ϊ1.84g/�M3��Ũ����______ mL����С�������1λ��Ч���֣� ��Һ����������ش��������⣺
��Һ����������ش��������⣺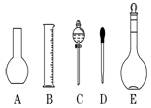
 ŨH2SO4������500 mL 0.2
ŨH2SO4������500 mL 0.2 mol /L��ϡH2SO4�������������ٲ���������Ͳ���ձ��ܽ�ͷ�ι�
mol /L��ϡH2SO4�������������ٲ���������Ͳ���ձ��ܽ�ͷ�ι� 00 mL����
00 mL���� �Ƶ�����ƿ֮ǰ��Ҫ�ָ�����������Ϊ_______________________________
�Ƶ�����ƿ֮ǰ��Ҫ�ָ�����������Ϊ_______________________________ .����ʱ������ˮ���������˿̶�_______________
.����ʱ������ˮ���������˿̶�_______________