��Ŀ����
(6��)��1����18.0 mol/L H2SO4����100 mL 1.00mol/L H2SO4������Ҫ��ʵ�����������ձ�������������ͷ�ι����Ӧ���У�______
���ƹ����У����������ʹ���ƽ��ƫ�ߵ���(�����)____
�ٶ����Ǹ��ӿ̶��߹۲�Һ��
������ƿʹ��ʱδ����
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶�
������ƿʢ��H2SO4��Һ��ʹ��ǰδϴ��
��2��������ƿʹ�÷����У����в�������ȷ����(����ĸ)_________
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô���Һ��ϴ
C ��������Һʱ�����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������������ˮ���ӽ�����1��2�M�����ٸ��ý�ͷ�ιܼ�����ˮ���̶���
��������Һʱ�����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������������ˮ���ӽ�����1��2�M�����ٸ��ý�ͷ�ιܼ�����ˮ���̶���
D���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ��,������ƿ��תҡ��
��1��100 mL ����ƿ����Ͳ��2�֣� �� ��___�� ��2�֣�(2)___B C___��2��
����

��ϰ��ϵ�д�
�����Ŀ
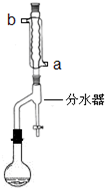 ��ƻ�����㽶��ˮ���Ĺ����д�����������������ij��ѧ������ȤС�����������������Ϊԭ�Ϻϳ�������������
��ƻ�����㽶��ˮ���Ĺ����д�����������������ij��ѧ������ȤС�����������������Ϊԭ�Ϻϳ�������������