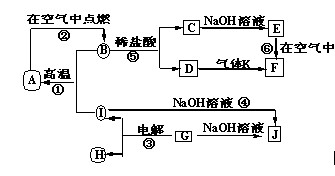
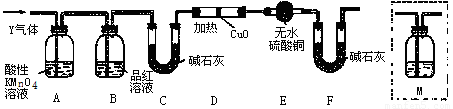
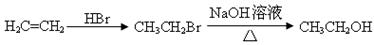��Ŀ����
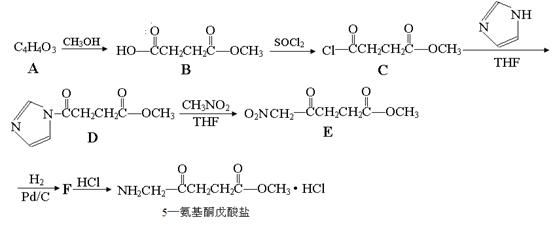
�����14�֣�5������ͪ��������һ�ֿ�����ҩ����ϳ�·�����£�
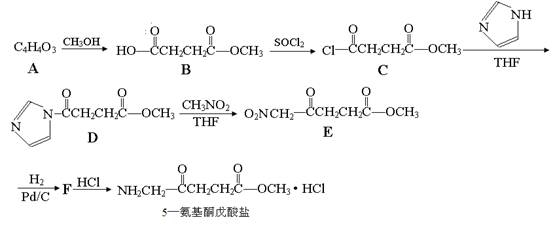
��֪��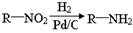
��1����֪A���ӽṹ����һ������A����ʹ��ˮ��ɫ���Һ˴Ź�������ͼ��ֻ��һ���壬��A�Ľṹ��ʽΪ ��
��2��5������ͪ�������к��������ŵ������� ��C��D�ķ�Ӧ����Ϊ ��
��3��G��B��һ��ͬ���칹�壬����NaHCO3��Һ��Ӧ���ܷ���������Ӧ��1molG����������Na��Ӧ������1molH2����G�����в�������д��һ�ַ�������������G�Ľṹ��ʽ ��
��4��д��D��E�ķ�Ӧ����ʽ ��
��5����֪ ����������������Ϣ��д����CH3CH2COOH��
����������������Ϣ��д����CH3CH2COOH��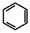 Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ�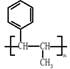 ����ĺϳ�·������ͼ�����Լ���ѡ����
����ĺϳ�·������ͼ�����Լ���ѡ����
�ϳ�·������ͼʾ�����£�
��
��1��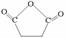 ��2�֣�
��2�֣�
��2���ʻ���������ȫ�Ե�2�֣�дһ������ȷ��1�֣����ִ�д��0�֣���ȡ����Ӧ��2�֣�
��3�� ����дһ����2�֣�
����дһ����2�֣�
��4�� ��2�֣�
��2�֣�
��5��
(4�֣���һ����ȫ�������������÷�)
��������(1)����A�����ʿ�֪��A�ĽṹӦ���ǶԳƵģ����Խṹ��ʽΪ ��
��
��2������5������ͪ�����εĽṹ��ʽ��֪�������к��������ŵ��������ʻ�������������CD�Ľṹ��ʽ��֪�������ﻹ��HCl�����൱���ǰ�������ԭ�ӱ�ȡ��������Ӧ����ȡ����Ӧ��
��3������NaHCO3��Һ��Ӧ���ܷ���������Ӧ�����Ի����Ȼ���ȩ��������Ϊ1molG����������Na��Ӧ������1molH2�����Ի�����1���ǻ�����˿��ܵĽṹ��ʽΪ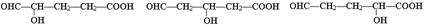 ��
��
��4������ԭ���غ��֪����Ӧ�ķ���ʽΪ
 ��
��
��5�������л���ĺϳɣ�һ��������Ʒ���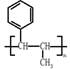 ���ڸ߷��ӻ�������Ǽ۲���䵥����
���ڸ߷��ӻ�������Ǽ۲���䵥����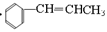 �����Ժϳ�·����
�����Ժϳ�·����
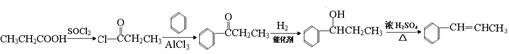 ��
��

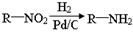
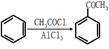 ����������������Ϣ��д����CH3CH2COOH��
����������������Ϣ��д����CH3CH2COOH�� Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ�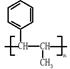 ����ĺϳ�·������ͼ�����Լ���ѡ����
����ĺϳ�·������ͼ�����Լ���ѡ����