��Ŀ����
(17��)
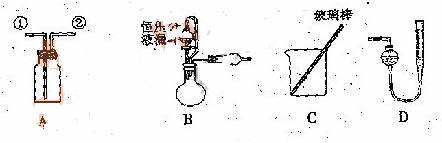
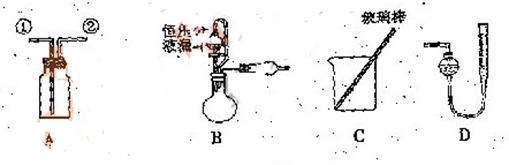
![]() ij�о���С�����A-D������װ������й�ʵ��
ij�о���С�����A-D������װ������й�ʵ��
![]()
![]() ��ʵ��һ���ռ�NO���塣
��ʵ��һ���ռ�NO���塣
��1������ ![]() ��װ��A�ռ�NO���壬��ȷ�IJ����������� ������ţ���
��װ��A�ռ�NO���壬��ȷ�IJ����������� ������ţ���
![]() a.�Ӣٿڽ���������ˮ�������� ��������������b.�Ӣٿڽ�����������������
a.�Ӣٿڽ���������ˮ�������� ��������������b.�Ӣٿڽ�����������������
![]() c.�Ӣڿڽ���������ˮ���������������������� d..�Ӣڿڽ�����������������
c.�Ӣڿڽ���������ˮ���������������������� d..�Ӣڿڽ�����������������
![]() ��ʵ�����Ϊ��̽����п�������ϵ�п����������
��ʵ�����Ϊ��̽����п�������ϵ�п����������![]() �ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
�ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na2ZnO3+H2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm1 g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�顣
![]() �����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
�����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�ꡣ
![]() ��2��ѡ��B���������� ����������ţ�����װ�ý���ʵ�顣
��2��ѡ��B���������� ����������ţ�����װ�ý���ʵ�顣
![]() ��3����ó�ַ�Ӧ���������������ΪVL����״������
��3����ó�ַ�Ӧ���������������ΪVL����״������![]() =������ ��
=������ ��
![]() ��4������Ʋ��ȣ�����Ҫ������һ������������������������ ��
��4������Ʋ��ȣ�����Ҫ������һ������������������������ ��
![]() ��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©����������������ƫ����ƫС������Ӱ�족����
![]() �����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�ꡣѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm2g ��
![]() ��6��
��6��![]() ���������� ��
���������� ��
![]() ��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH2��������ʵ��̽��Ŀ�ꡣʵ��ͬ��ʹ������C��
![]() ��7����ʵ�����Ƕȷ������������������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
��7����ʵ�����Ƕȷ������������������� �����ң�����ڡ��������ڡ���ͬ�ڡ�����
��(1)C (2)D (3) ![]() (��
(��![]() �����������𰸣�
�����������𰸣�
��4������п���ܶȣ������������𰸣�
��5��ƫ��
��6��![]() �������������𰸣�
�������������𰸣�
��7������
�����������⿼��ʵ���̽�����漰NO������ռ���������н��������ļ���ȡ���1��NO�������������������ʲ������ſ������ռ���ֻ������ˮ���ռ�����ˮʱӦ�ö̽���������2�������ף�Zn��Fe��ֻ��Zn������NaOH�������壬ͨ����ˮ�ռ����壬���ݷ�Ӧ����ʽ�������Zn������������������Ҫ��װ���в�H2�������Dװ�á���3��Zn��H2֮����Ϊ1��1����n(Zn)=V/22.4 mol,w (Zn)=m(Zn)/m1 = ![]() ����4������Zn��������������ܶȣ���������Zn�����������Zn�Ľ�������������Zn�ĸ߶ȣ���ȣ�����5����ѹʽ��Һ©�������������в��ֲ����ڷ�Һ©���Ϸ���������ʱ�ռ����ˣ���������ͨ©��ʱ�ռ���H2��һЩ����������Zn����ƫ��6�����ٵ�������ΪZn����������7������������H2��������ֵ����Ȼ������Ϊ������H2������С������ƫ���
����4������Zn��������������ܶȣ���������Zn�����������Zn�Ľ�������������Zn�ĸ߶ȣ���ȣ�����5����ѹʽ��Һ©�������������в��ֲ����ڷ�Һ©���Ϸ���������ʱ�ռ����ˣ���������ͨ©��ʱ�ռ���H2��һЩ����������Zn����ƫ��6�����ٵ�������ΪZn����������7������������H2��������ֵ����Ȼ������Ϊ������H2������С������ƫ���

 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�