��Ŀ����
��16�֣���(Pd)�������벬���ơ���ҵ�ϴӷϴ�������Ҫ�ɷ����ٺͻ���̿����������������п���л����٣������������̣�
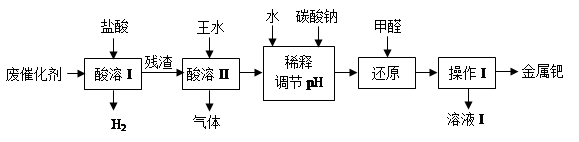
��ش��������⣺
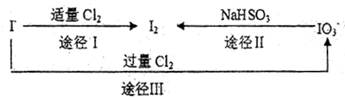
��1������I��Ŀ����__________________________________��
��2�������ܢ�ʱ��������ˮ�ڼ���������������Ҫ��Ӧ�ǣ�
3Pd +12HCl + 2HNO3 3H2PdCl4 + 2NO��+ 4H2O
3H2PdCl4 + 2NO��+ 4H2O
д����������һ��Ҫ�ɷ���Ũ���ᷴӦ�Ļ�ѧ����ʽ��_________________��
�����ܢ�������¶Ȳ��˹��ߣ����˿���һ����Ӧ�����⣬��ԭ����ܻ���
_____________________________��
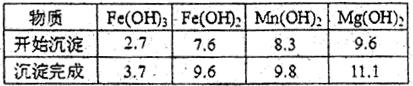
�������������ռ��������壬��д�����պ���Һ�к��е����ʵĻ�ѧʽ��NaOH��NaNO2��___________��___________��
��3��ʹ�ü�ȩ��ԭ�ٵĻ�����ʱ����Һ�뱣�ּ��ԣ��������ɼ�ȩ�Ķ�����ģ�ԭ����_______________________________________��
��4������I��������_______________����ҺI���ܺ��е��л�����Ϊ________________��
��5������������ڽ�������ǰ����Ƚ��ϴ�����700���½������գ�ͬʱ����ͨ�������
��Ŀ����______________________________________________________��
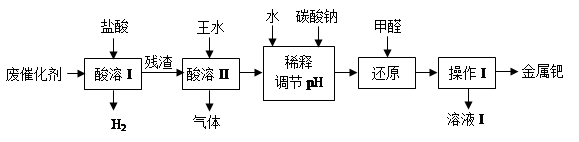
��ش��������⣺
��1������I��Ŀ����__________________________________��
��2�������ܢ�ʱ��������ˮ�ڼ���������������Ҫ��Ӧ�ǣ�
3Pd +12HCl + 2HNO3
 3H2PdCl4 + 2NO��+ 4H2O
3H2PdCl4 + 2NO��+ 4H2Oд����������һ��Ҫ�ɷ���Ũ���ᷴӦ�Ļ�ѧ����ʽ��_________________��
�����ܢ�������¶Ȳ��˹��ߣ����˿���һ����Ӧ�����⣬��ԭ����ܻ���
_____________________________��
�������������ռ��������壬��д�����պ���Һ�к��е����ʵĻ�ѧʽ��NaOH��NaNO2��___________��___________��
��3��ʹ�ü�ȩ��ԭ�ٵĻ�����ʱ����Һ�뱣�ּ��ԣ��������ɼ�ȩ�Ķ�����ģ�ԭ����_______________________________________��
��4������I��������_______________����ҺI���ܺ��е��л�����Ϊ________________��
��5������������ڽ�������ǰ����Ƚ��ϴ�����700���½������գ�ͬʱ����ͨ�������
��Ŀ����______________________________________________________��
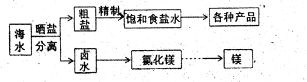
��1����ȥ����п������ ��2�֣�
��2����C+4HNO3��Ũ�� CO2��+4NO2��+2H2O ��2�֣�
CO2��+4NO2��+2H2O ��2�֣�
�ڷ�ֹ����ֽ⣬��ֹ���ᡢ����ӷ���2�֣�
��NaNO3��Na2CO3��2�֣�
��3�����������£���ȩ�ᱻ����������2�֣� ��4�����ˣ�2�֣� HCOO?��2�֣�
��5����ȥ�ϴ����еĻ���̿��������ˮ�����ģ�2�֣�
��2����C+4HNO3��Ũ��
 CO2��+4NO2��+2H2O ��2�֣�
CO2��+4NO2��+2H2O ��2�֣��ڷ�ֹ����ֽ⣬��ֹ���ᡢ����ӷ���2�֣�
��NaNO3��Na2CO3��2�֣�
��3�����������£���ȩ�ᱻ����������2�֣� ��4�����ˣ�2�֣� HCOO?��2�֣�
��5����ȥ�ϴ����еĻ���̿��������ˮ�����ģ�2�֣�
��1�����ں���п�����������ữ��Ŀ���dz�ȥп������
��2����Ũ�������ǿ�����ԣ�Ҳ����������̼����ӦʽΪC+4HNO3��Ũ�� CO2��+4NO2��+2H2O��
CO2��+4NO2��+2H2O��
�����������ֽ⣬����������ᶼ�ӷ��������¶Ȳ���̫�ߡ�
����Ϊ�����к���CO2��NO2�����Ի������̼���ƺ������ơ�
��3�����������������£�������������ȩ��������Һ���뱣�ּ��ԡ�
��4������Pd������ˮ�����˼��ɡ���ȩ�����������Ǽ��ᣬ���Կ��ܺ��е��л������� HCOO?��
��5����Ϊ�ڼ���ʱ̼�ܱ�������������CO2���������Լ�����ˮ�����ġ�
��2����Ũ�������ǿ�����ԣ�Ҳ����������̼����ӦʽΪC+4HNO3��Ũ��
 CO2��+4NO2��+2H2O��
CO2��+4NO2��+2H2O�������������ֽ⣬����������ᶼ�ӷ��������¶Ȳ���̫�ߡ�
����Ϊ�����к���CO2��NO2�����Ի������̼���ƺ������ơ�
��3�����������������£�������������ȩ��������Һ���뱣�ּ��ԡ�
��4������Pd������ˮ�����˼��ɡ���ȩ�����������Ǽ��ᣬ���Կ��ܺ��е��л������� HCOO?��
��5����Ϊ�ڼ���ʱ̼�ܱ�������������CO2���������Լ�����ˮ�����ġ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �ķ���ұ��
�ķ���ұ�� ��Һ����ұ��
��Һ����ұ��