��Ŀ����
����Ŀ����ͼ��ʾ��һ�ܱ���������Ħ�����ɻ�����������a��b�ֳɼס������ң���״���£��������г���NH3 0.4mol�������г���HCl��N2�Ļ�����壬��ֹʱ����λ����ͼ��ʾ����֪�ס��������������������Ϊ17.3g��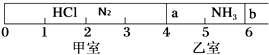
��1�����������������Ϊ ��
��2��������HCl��N2�����ʵ���֮��Ϊ ��
��3��������aȥ������HCl��NH3��ַ�Ӧ����NH4Cl����������˷�Ӧ��������b��λ�ڿ̶ȡ������������֣������ǹ������ʲ�����ѹǿ������ʱ��ϵ��ƽ��Ħ������ ��
���𰸡�
��1��24.1g
��2��1��3
��3��4��25.25g/mol
���������⣺��1�������г���NH3 �� ����������Ϊm=nM=0.4mol��17g/mol=6.8g����֪�ס��������������������Ϊ17.3g����������������Ϊ17.3g+6.8g=24.1g���ʴ�Ϊ��24.1g����2����ͬ�����£���������ʵ���֮�ȵ��������֮�ȣ���ͼ��֪�ס�����������������Ϊ2��1���������ʵ���֮��Ϊ2��1���������г���NH3 0.4mol�����Լ���������Ϊ0.8mol���������������Ϊ24.1g����HCl�����ʵ���Ϊx�����������ʵ���Ϊy�����������ʵ����������з�����Ϊ�� ![]() �����x=0.2mol��y=0.6mol������HCl��N2�����ʵ���֮��Ϊ0.2mol��0.6mol=1��3���ʴ�Ϊ��1��3����3��������NH3�����ʵ���Ϊ0.4mol������HCl�����ʵ���Ϊ0.2mol�����Է�Ӧ����NH4Cl���壬ʣ��NH3�����ʵ���Ϊ0.2mol��ʣ������������ʵ���Ϊ0.8mol����ͬ�����£���������֮�ȵ��������ʵ���֮�ȣ���ʼʱ���������ʵ���Ϊ1.2mol�������b��6�����������������ʵ���Ϊ0.8mol�����Ի���b������������4��������ϵ��ƽ��Ħ������M=
�����x=0.2mol��y=0.6mol������HCl��N2�����ʵ���֮��Ϊ0.2mol��0.6mol=1��3���ʴ�Ϊ��1��3����3��������NH3�����ʵ���Ϊ0.4mol������HCl�����ʵ���Ϊ0.2mol�����Է�Ӧ����NH4Cl���壬ʣ��NH3�����ʵ���Ϊ0.2mol��ʣ������������ʵ���Ϊ0.8mol����ͬ�����£���������֮�ȵ��������ʵ���֮�ȣ���ʼʱ���������ʵ���Ϊ1.2mol�������b��6�����������������ʵ���Ϊ0.8mol�����Ի���b������������4��������ϵ��ƽ��Ħ������M= ![]() =
= ![]() =25.25g/mol��
=25.25g/mol��
�ʴ�Ϊ��4��25.25g/mol��
��1�������г���NH3 �� ����m=nM���������������������������������������������2�����ݼ�������������ʵ�������������HCl�͵��������ʵ���֮�ȣ���3������ʣ����������ʵ�������ʣ��������ռ������Ӷ�ȷ��b��λ�ã�����M= ![]() ���㣮
���㣮

 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�