��Ŀ����
(8��)(2011������ģ��)��֪A��B��֧�Թܵ���Һ�й�����K����Ag����Mg2����Cl����OH����NO3���������ӡ����Թ�A����Һ�е����̪��Һ�ʷۺ�ɫ����ش��������⣺
(1)�Թ�A����Һ�������������ӹ���________�֡�
(2)����ij�Թ��е���ϡ�����������������Թ�Ϊ________(�A����B��)��
(3)�����Թ�B����Һ�м�����ʵ�ҩƷ�����˺���Եõ���Ӧ�Ľ����ͽ���һ�����ʵ���Һ��������ҩƷ��________(�ѧʽ)��
(4)�����Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺�������Һ�ɵõ�һ�ִ�������Ϲ����з�����Ӧ�����ӷ���ʽΪ
________________________________________________________________________��______________________________________________________________________��
(5)���Թ�A���Թ�B�й����������ʰ������ʵ����ܽ����Թ��У��ٽ�A��B�е���Һ��Ϲ��ˣ�������Һ�и������ӵ����ʵ���֮��Ϊ________��
(6)�������Թ�A����Һ����������ɵ�̼��������Һ�У���������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ________________________________________________________��
(1)�Թ�A����Һ�������������ӹ���________�֡�
(2)����ij�Թ��е���ϡ�����������������Թ�Ϊ________(�A����B��)��
(3)�����Թ�B����Һ�м�����ʵ�ҩƷ�����˺���Եõ���Ӧ�Ľ����ͽ���һ�����ʵ���Һ��������ҩƷ��________(�ѧʽ)��
(4)�����Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺�������Һ�ɵõ�һ�ִ�������Ϲ����з�����Ӧ�����ӷ���ʽΪ
________________________________________________________________________��______________________________________________________________________��
(5)���Թ�A���Թ�B�й����������ʰ������ʵ����ܽ����Թ��У��ٽ�A��B�е���Һ��Ϲ��ˣ�������Һ�и������ӵ����ʵ���֮��Ϊ________��
(6)�������Թ�A����Һ����������ɵ�̼��������Һ�У���������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ________________________________________________________��
(1)3��(2)B��(3)Mg
(4)Ag����Cl��===AgCl��
Mg2����2OH��===Mg(OH)2��
(5)n(K��) ��n(Mg2��) ��n(NO3��)��4 ��1 ��6
(6)Ba2����2OH����2HCO3��===BaCO3����CO32����2H2O
(4)Ag����Cl��===AgCl��
Mg2����2OH��===Mg(OH)2��
(5)n(K��) ��n(Mg2��) ��n(NO3��)��4 ��1 ��6
(6)Ba2����2OH����2HCO3��===BaCO3����CO32����2H2O
���Թ�A����Һ�е����̪��Һ�ʷۺ�ɫ��˵����Һ�Լ��ԣ�һ������OH�����������ӹ���ԭ����һ��û��Ag����Mg2����һ������K�����Թ�B��һ������Ag����Mg2������һ��û��Cl����һ������NO3���������������Թ�A�к���K����OH����Cl�����Թ�B�к���Ag����Mg2����NO3����
(1)�Թ�A����Һ�������������ӹ���3�֡�
(2)����ij�Թ��е���ϡ�����������������Թ��к���Ag����Ϊ�Թ�B��
(3)�����Թ�B����Һ�м�����ʵ�ҩƷ���˺���Һ�н�����һ�����ʣ��ҵõ���Ӧ�Ľ�����Ӧ�÷����û���Ӧ���Ҳ������������ӣ�������ҩƷ��Mg��
(4)�����Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺�������Һ�ɵõ�һ�ִ������Ag����Cl��ǡ����ȫ��Ӧ��Mg2����OH��ǡ����ȫ��Ӧ����Ϲ����з�����Ӧ�����ӷ���ʽΪ
Ag����Cl��===AgCl����
Mg2����2OH��===Mg(OH)2����
(5)��KOH��KCl��Mg(NO3)2��AgNO3�������ʾ�Ϊ1 mol���ܽ����Թ��У����˺�������Һ�к���2 mol K����0.5 mol Mg2����3 mol NO3����
(6)��KHCO3��Һ�У���������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪBa2����2OH����2HCO3��===BaCO3����CO32����2H2O��
(1)�Թ�A����Һ�������������ӹ���3�֡�
(2)����ij�Թ��е���ϡ�����������������Թ��к���Ag����Ϊ�Թ�B��
(3)�����Թ�B����Һ�м�����ʵ�ҩƷ���˺���Һ�н�����һ�����ʣ��ҵõ���Ӧ�Ľ�����Ӧ�÷����û���Ӧ���Ҳ������������ӣ�������ҩƷ��Mg��
(4)�����Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺�������Һ�ɵõ�һ�ִ������Ag����Cl��ǡ����ȫ��Ӧ��Mg2����OH��ǡ����ȫ��Ӧ����Ϲ����з�����Ӧ�����ӷ���ʽΪ
Ag����Cl��===AgCl����
Mg2����2OH��===Mg(OH)2����
(5)��KOH��KCl��Mg(NO3)2��AgNO3�������ʾ�Ϊ1 mol���ܽ����Թ��У����˺�������Һ�к���2 mol K����0.5 mol Mg2����3 mol NO3����
(6)��KHCO3��Һ�У���������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪBa2����2OH����2HCO3��===BaCO3����CO32����2H2O��

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�
�����Ŀ

 ��C��D��E�������Σ����Ƿֱ���
��C��D��E�������Σ����Ƿֱ���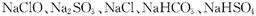 �е�ijһ�֡�Ϊ��֤��������
�е�ijһ�֡�Ϊ��֤�������� �ľ���ɷ�.��������Ʒ�ֱ������Һ�����ʵ��(����ʵ�鲻����ʹ�������Լ�)��
�ľ���ɷ�.��������Ʒ�ֱ������Һ�����ʵ��(����ʵ�鲻����ʹ�������Լ�)�� 1)ijͬѧ����������ʵ��.�����ʵ�����ݡ�
1)ijͬѧ����������ʵ��.�����ʵ�����ݡ�