��Ŀ����
�����ķ�չ�봴������ֹ�����ҹ�����ר�Һ�°�ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ����ͼ��
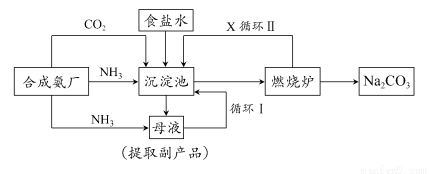
(1)���������Ҫͨ��CO2�Ͱ�����Ӧ��ͨ��________(�ѧʽ)��ԭ����________________��
(2)�������з����Ļ�ѧ��Ӧ����ʽ��________________��
(3)ĸҺ�е�������Ҫ��________����ĸҺ��ͨ�������ټ���ϸСʳ�ο�������ȴ��������Ʒ��ͨ�백����������________________��
(4)ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϣ���Ҫ�������________(�����������еı��)��ѭ��������X��________���ӳ���������ȡ�����IJ�����________________��
(5)д������¯�з�����Ӧ�Ļ�ѧ����ʽ____________________________��
(6)�����ƵõIJ�Ʒ̼�����п��ܺ��е�������________(�ѧʽ)��Ϊ��������ʵĴ��ڣ����������__________________________________��
(1)NH3����ΪCO2�ܽ�Ƚ�С����NH3������ˮ����ͨNH3������CO2����
(2)NH3��CO2��NaCl��H2O=NaHCO3����NH4Cl
(3)NH4Cl������NH4+Ũ�ȣ�ʹNH4Cl��������
(4)ѭ������CO2������
(5)2NaHCO3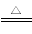 Na2CO3��CO2����H2O
Na2CO3��CO2����H2O
(6)NaCl��ȡ���������ܽ⣬����HNO3�ữ��AgNO3��Һ�����а�ɫ������˵������NaCl����
�����������������ͨ�백����ʹ��Һ�Լ��ԣ�������CO2���գ�ĸҺ�е�������Ҫ�Ǹ���ƷNH4Cl��ͨ����������ϸСʳ�ο�������ʹNH4Cl���ܽ�ƽ����ᾧ�����ƶ�����ȡѭ�����������ԭ�ϵ������ʣ��������˱���ʳ��ˮʹ������̼�����ƺ��Ȼ��ƣ����к��Ȼ��ơ�

 ��У����ϵ�д�
��У����ϵ�д�