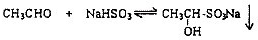��Ŀ����
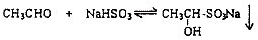
��1���ʻ�������ͱ���NaHSO3�ķ�Ӧ�������£�
| �ʻ������� | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ���ʣ�1Сʱ�ڣ� | 88.7 | 56.2 | 36.4 | 23.4 |
| �ʻ������� | ��CH3��2CHCOCH3 | ��CH3��3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ���ʣ�1Сʱ�ڣ� | 12.3 | 5.6 | 2 | 1 |
��______��
��______��
��______��
��2���������Ͽ��淴Ӧ���Է���ȩ��ͪ�Ļ�����д����ʹȩ��NaHSO3���ɵij��������ܽ���Լ��Ļ�ѧʽ______��д��2�֣����ڲ�ͬ�������ʣ�
��3�������ͪ��RCOCH3��ͨ���õ�����2����Ӧ������������ˮ���ȷ£�
��CH3��2CHCOCH3
| Cl2NaOH |
| NaOH |
| �� |
��д����һ����Ӧ�Ļ�ѧ����ʽ______��
��д��A�Ľṹ��ʽ______��
��4������ȩ����ŨNaOH��Һ��Ӧ���ɱ������ƺͱ��״����˷�Ӧ��������______������÷�Ӧ����IJ���������______��
CH3COCH3��C6H5COCH3��ȣ�CH3COCH3��Ӧ�IJ��ʴ���C6H5COCH3��Ӧ�IJ��ʣ��ɴ˵ó�����ͪ���ѷ����÷�Ӧ��
CH3COCH3��C2H5COCH3��CH3CH2CH2COCH3 ��ȣ���Ӧ��������ȡ������̼ԭ����Խ������٣�˵��Խ�ѷ�Ӧ��
CH3CH2CH2COCH3����CH3��2CHCOCH3����CH3��3CCOCH3��ȣ���Ӧ���������ʻ�������̼����ԭ��Խ�ٻ�ȡ����Խ������٣�˵��Խ�ѷ�Ӧ��
�ʴ�Ϊ��ȩ��ͪ�������÷�Ӧ������ͪ���ѷ����÷�Ӧ���ʻ�������̼����ԭ��Խ�ٻ�ȡ����Խ���ȡ������̼ԭ����Խ�࣬Խ�ѷ�Ӧ��
��2���ʻ��������뱥��NaHSO3��Һ�ķ�Ӧ�ǿ��淴Ӧ�����ʹ�����ܽ⣬ƽ��Ӧ���淴Ӧ�����ƶ���ֻҪ�������������Ƶ�Ũ�ȼ��ɣ�����������ǿ��������ʽ�Σ��ܺ�ǿ�ᡢǿ�Ӧ�����Կ�����Һ�м���������������ƣ�ʹƽ�����淴Ӧ�����ƶ����ﵽ�ܽ������Ŀ�ģ�
�ʴ�Ϊ��HCl��NaOH��
��3���٣�CH3��2CHCOCH3
| Cl2NaOH |
ͨ����Ӧ��Ͳ���������֪����CH3��2CHCOCH3����������ȡ����Ӧ���ɣ�CH3��2CHCOCCl3���Ȼ��⣬�Ȼ�����������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��������������ˣ�CH3��2CHCOCCl3�����Ȼ��ƺ�ˮ��
��Ӧ����ʽΪ����CH3��2CHCOCH3+3Cl2+3NaOH����CH3��2CHCOCCl3+3NaCl+3H2O��
�ʴ�Ϊ����CH3��2CHCOCH3+3Cl2+3NaOH����CH3��2CHCOCCl3+3NaCl+3H2O��
�ڣ�CH3��2CHCOCCl3
| NaOH |
| �� |
�ʴ�Ϊ����CH3��2CHCOONa��
��4���ަ�-H��ȩ��Ũ����Һ�������ܷ����绯��Ӧ��������ʹ����˷�Ӧ�ʿ�������Ӧ���÷�ӦҲ��������ԭ��Ӧ����������������ˮ�����״���������ˮ�����Կ��Ը����ܽ�ȵIJ�ͬ��ȡ��ȡ�ķ������룬Ҳ�����÷е�IJ�ͬ��������ķ������룮
�ʴ�Ϊ��������ԭ��Ӧ ���绯��Ӧ ������ȡ��������

��֪�ʻ��������뱥��NaHSO3��Һ���Է������·�Ӧ��
![]()
��1���ʻ�������ͱ���NaHSO3�ķ�Ӧ�������£�
�ʻ������� | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
����(1Сʱ��) | 88.7 | 56.2 | 36.4 | 23.4 |
�ʻ������� | (CH3)2CHCOCH3 | (CH3)3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
����(1Сʱ��) | 12.3 | 5.6 | 2 | 1 |
�ɼ���ȡ�������ʻ��������NaHSO3��Ӧ��Ӱ���У�д��3�����ɣ�
���������� ��������������������������
![]() ���������������� ������������������
���������������� ������������������
![]() ���������������� �������������� ����
���������������� �������������� ����
![]() ��2���������Ͽ��淴Ӧ���Է���ȩ��ͪ�Ļ�����д����ʹȩ��NaHSO3���ɵij��������ܽ���Լ��Ļ�ѧʽ����������������������������д��2�֣����ڲ�ͬ�������ʣ�
��2���������Ͽ��淴Ӧ���Է���ȩ��ͪ�Ļ�����д����ʹȩ��NaHSO3���ɵij��������ܽ���Լ��Ļ�ѧʽ����������������������������д��2�֣����ڲ�ͬ�������ʣ�
![]() ��3�������ͪ(RCOCH3 )ͨ���õ�����2����Ӧ������������ˮ���ȷ¡�
��3�������ͪ(RCOCH3 )ͨ���õ�����2����Ӧ������������ˮ���ȷ¡�
![]()
![]()
![]()
![]()
��д����һ����Ӧ�Ļ�ѧ����ʽ������������������������ ����
��д��A�Ľṹ��ʽ������������������������������
��4������ȩ����ŨNaOH��Һ��Ӧ���ɱ������ƺͱ��״����˷�Ӧ���������� ��������
����÷�Ӧ����IJ����������������� ����������������������

��1���ʻ�������ͱ���NaHSO3�ķ�Ӧ�������£�
| �ʻ������� | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ���ʣ�1Сʱ�ڣ� | 88.7 | 56.2 | 36.4 | 23.4 |
| �ʻ������� | ��CH3��2CHCOCH3 | ��CH3��3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ���ʣ�1Сʱ�ڣ� | 12.3 | 5.6 | 2 | 1 |
��______��
��______��
��______��
��2���������Ͽ��淴Ӧ���Է���ȩ��ͪ�Ļ�����д����ʹȩ��NaHSO3���ɵij��������ܽ���Լ��Ļ�ѧʽ______��д��2�֣����ڲ�ͬ�������ʣ�
��3�������ͪ��RCOCH3��ͨ���õ�����2����Ӧ������������ˮ���ȷ£�
��CH3��2CHCOCH3
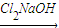 ��CH3��2CHCOCCl3
��CH3��2CHCOCCl3 A+HCCl3��
A+HCCl3����д����һ����Ӧ�Ļ�ѧ����ʽ______��
��д��A�Ľṹ��ʽ______��
��4������ȩ����ŨNaOH��Һ��Ӧ���ɱ������ƺͱ��״����˷�Ӧ��������______������÷�Ӧ����IJ���������______��
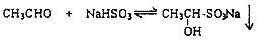
��1���ʻ�������ͱ���NaHSO3�ķ�Ӧ�������£�
| �ʻ������� | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ���ʣ�1Сʱ�ڣ� | 88.7 | 56.2 | 36.4 | 23.4 |
| �ʻ������� | ��CH3��2CHCOCH3 | ��CH3��3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ���ʣ�1Сʱ�ڣ� | 12.3 | 5.6 | 2 | 1 |
��______��
��______��
��______��
��2���������Ͽ��淴Ӧ���Է���ȩ��ͪ�Ļ�����д����ʹȩ��NaHSO3���ɵij��������ܽ���Լ��Ļ�ѧʽ______��д��2�֣����ڲ�ͬ�������ʣ�
��3�������ͪ��RCOCH3��ͨ���õ�����2����Ӧ������������ˮ���ȷ£�
��CH3��2CHCOCH3
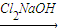 ��CH3��2CHCOCCl3
��CH3��2CHCOCCl3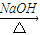 A+HCCl3��
A+HCCl3����д����һ����Ӧ�Ļ�ѧ����ʽ______��
��д��A�Ľṹ��ʽ______��
��4������ȩ����ŨNaOH��Һ��Ӧ���ɱ������ƺͱ��״����˷�Ӧ��������______������÷�Ӧ����IJ���������______��