��Ŀ����
(10��)���ú��������Ȼ��Ƶ��Ȼ��ƹ��壬����100 mL 1mol/L���Ȼ�����Һ�����������IJ������������ݷ�����������ش��������⣺
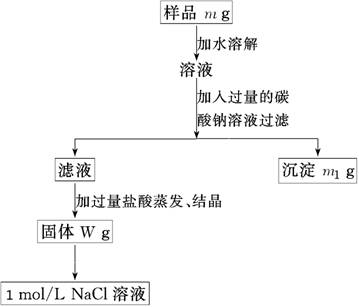
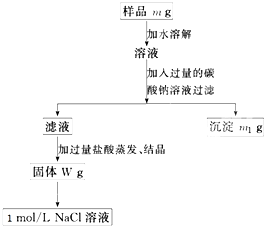
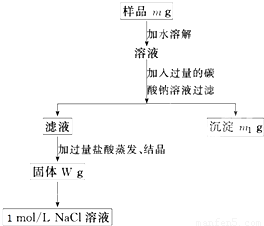
�ų���������Ʒm g����Ʒ����������ƽ�����ϡ�
�ƹ���ʱ�����õ��IJ����������ձ������������� ��
����������ʱӦ��Һ�����___ ___�м��ȣ��ȼ�����____ ____ʱ��ֹͣ���ȡ�
������Ʒ����Һ�м��������Na2CO3��Һ��������____________________________����Ӧ�����ӷ���ʽ��___ ___________________��
������Һ�м��������������________ ________��������Ӧ�Ļ�ѧ����ʽ��____ _______��
������100 mL 1mol/L��NaCl��Һʱ��Ӧ��W g�����г�ȡNaCl������Ϊ____________��
(10�֣�ÿ��1��,����ʽ��2��) (2)©�� (3)������ʣ�����Һ�塡
(4)ʹ��������ȫ�γɳ�������ȥ��Ca2+��CO32��-===CaCO3��
(5)��ȥ������Na2CO3 Na2CO3��2HCl===2NaCl��H2O��CO2��
(6)5.9 g
��������
�����������2������ʱ�����õ��IJ����������ձ�������������©����
��3����������ʱӦ��Һ������������м��ȣ��ȼ�����ʣ�����Һ��ʱ��ֹͣ���ȡ�
��4���Ȼ����к����Ȼ��ƣ���������Ʒ����Һ�м��������Na2CO3��Һ��������ʹ��������ȫ�γɳ�������ȥ����Ӧ�����ӷ���ʽ��Ca2+��CO32��-===CaCO3����
��5������̼�����ǹ����ģ���������Һ�м�������������dz�ȥ������Na2CO3���йط�Ӧ�Ļ�ѧ����ʽ��Na2CO3��2HCl===2NaCl��H2O��CO2����
��6��100 mL 1mol/L��NaCl��Һֻ���ʵ�������0.1L��0.1mol/L��58.5g/mol��5.85g������������ƽֻ�ܶ�����0.1g������ʵ�ʳ�����������5.9g��
���㣺���鳣���Ļ���ʵ�������
��������ѧʵ�鳣��������ʹ�÷����ͻ�ѧʵ����������ǽ��л�ѧʵ��Ļ������Ի�ѧʵ��Ŀ����벻����ѧʵ��Ļ������������Ա�����������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ���ú��������Ȼ��Ƶ��Ȼ��ƹ��壬����100mL 1mol/L���Ȼ�����Һ�����������IJ������������ݷ�����������ش��������⣺
���ú��������Ȼ��Ƶ��Ȼ��ƹ��壬����100mL 1mol/L���Ȼ�����Һ�����������IJ������������ݷ�����������ش��������⣺