��Ŀ����
���ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС������ʵ������ģ���������̣�����Ƶ�ģ��װ�����£� ��ʾ��ʵ������Cl2�Ļ�ѧ��Ӧ����ʽΪMnO2 + 4HCl��Ũ�� == MnCl2 + Cl2��+ H2O
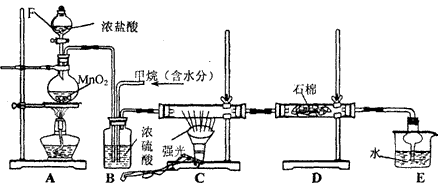
����Ҫ����գ�
(1) д��CH4��Cl2����һ�ȴ���Ļ�ѧ��Ӧ����ʽ_____________��
(2) Bװ�������ֹ��ܣ��ٿ����������٣��ڽ������Ͼ��ȣ���________________��
(3) Dװ���е�ʯ���Ͼ��ȸ���KI��ĩ����������_______________��
(4) Eװ�õ�������____________(����)��
A���ռ����� B���������� C����ֹ���� D�������Ȼ���
(5) װ���г��������������⣬�������л����E�з�����������ѷ���Ϊ__________��
(6) ��װ�û���ȱ��,ԭ����û�н���β������,��β������Ҫ�ɷ���____________(����)��
A��CH4 B��CH3Cl C��CH2Cl2 D��CHCl3 E��CCl4
(1) д��CH4��Cl2����һ�ȴ���Ļ�ѧ��Ӧ����ʽ_____________��
(2) Bװ�������ֹ��ܣ��ٿ����������٣��ڽ������Ͼ��ȣ���________________��
(3) Dװ���е�ʯ���Ͼ��ȸ���KI��ĩ����������_______________��
(4) Eװ�õ�������____________(����)��
A���ռ����� B���������� C����ֹ���� D�������Ȼ���
(5) װ���г��������������⣬�������л����E�з�����������ѷ���Ϊ__________��
(6) ��װ�û���ȱ��,ԭ����û�н���β������,��β������Ҫ�ɷ���____________(����)��
A��CH4 B��CH3Cl C��CH2Cl2 D��CHCl3 E��CCl4
(1) CH4+ Cl2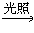 CH3Cl+HCl
CH3Cl+HCl
(2)����������
(3)��ȥ����������
(4) C D
(5)��Һ
(6)A B
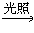 CH3Cl+HCl
CH3Cl+HCl(2)����������
(3)��ȥ����������
(4) C D
(5)��Һ
(6)A B

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 ?
?
