��Ŀ����
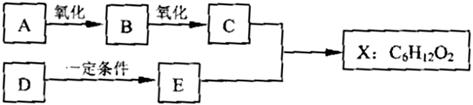
��ͼ��X����֧���ġ����й���ζ�ĺϳ����ϣ������ڵ�����ֹ������㾫����֪D�ڱ�״���µ��ܶ�Ϊ1.25g/L�������������������һ������ʯ�ͻ�����չˮƽ��E�������г�����һ���л�������ʼ�ת����ϵ���£�

��ش��������⣮
��1��A��������
��2��B��������������
��3��C+E��X�Ļ�ѧ��Ӧ������
��4��д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ ������A����
��5��X������������Һ��Ӧ�Ļ�ѧ����ʽ��
��6����DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ��
 ��
��

��ش��������⣮
��1��A��������
1-����
1-����
����2��B��������������
ȩ��
ȩ��
����3��C+E��X�Ļ�ѧ��Ӧ������
������ȡ����
������ȡ����
��Ӧ����4��д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ ������A����
C��CH3��3OH
C��CH3��3OH
��CH3CH2CH��OH��CH3
CH3CH2CH��OH��CH3
����5��X������������Һ��Ӧ�Ļ�ѧ����ʽ��
CH3CH2CH2COOCH2CH3+NaOH
CH3CH2CH2COONa+CH3CH2OH
| �� |
CH3CH2CH2COOCH2CH3+NaOH
CH3CH2CH2COONa+CH3CH2OH
��| �� |
��6����DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ��


������D�ڱ�״���µ��ܶ�Ϊ1.25g/L������Է�������Ϊ1.25��22.4=28�������������������һ������ʯ�ͻ�����չˮƽ��ӦΪCH2=CH2��A�������������ֱ�����B��C����AӦΪ����BΪȩ��CΪ�ᣬ��X����֧���ġ����й���ζ�ĺϳ����ϣ�ӦΪ��������EΪ����ӦΪCH3CH2OH����XΪCH3CH2CH2COOCH2CH3��
CΪCH3CH2CH2COOH��BΪCH3CH2CH2CHO��AΪCH3CH2CH2CH2OH����϶�Ӧ�л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
CΪCH3CH2CH2COOH��BΪCH3CH2CH2CHO��AΪCH3CH2CH2CH2OH����϶�Ӧ�л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
����⣺D�ڱ�״���µ��ܶ�Ϊ1.25g/L������Է�������Ϊ1.25��22.4=28�������������������һ������ʯ�ͻ�����չˮƽ��ӦΪCH2=CH2��A�������������ֱ�����B��C����AӦΪ����BΪȩ��CΪ�ᣬ��X����֧���ġ����й���ζ�ĺϳ����ϣ�ӦΪ��������EΪ����ӦΪCH3CH2OH����XΪCH3CH2CH2COOCH2CH3��
CΪCH3CH2CH2COOH��BΪCH3CH2CH2CHO��AΪCH3CH2CH2CH2OH��
��1��AΪCH3CH2CH2CH2OH��Ϊ1-�������ʴ�Ϊ��1-������
��2��BΪCH3CH2CH2CHO�����еĹ�����Ϊȩ�����ʴ�Ϊ��ȩ����
��3�������Ϸ�����֪C+E��X�Ļ�ѧ��Ӧ������������ȡ������Ӧ��
�ʴ�Ϊ��������ȡ������
��4��AΪCH3CH2CH2CH2OH����Ӧ��ͬ���칹��C��CH3��3OH��CH3CH2CH��OH��CH3��CH3C��CH3��CH2OH�ȣ�
�ʴ�Ϊ��C��CH3��3OH��CH3CH2CH��OH��CH3��CH3C��CH3��CH2OH��������д��������
��5��XΪCH3CH2CH2COOCH2CH3����NaOH��Һ�ɷ���ˮ�⣬��Ӧ�ķ���ʽΪCH3CH2CH2COOCH2CH3+NaOH
CH3CH2CH2COONa+CH3CH2OH��
�ʴ�Ϊ��CH3CH2CH2COOCH2CH3+NaOH
CH3CH2CH2COONa+CH3CH2OH��
��6��DΪCH2=CH2���ɷ����Ӿ۷�Ӧ����Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ��
CΪCH3CH2CH2COOH��BΪCH3CH2CH2CHO��AΪCH3CH2CH2CH2OH��
��1��AΪCH3CH2CH2CH2OH��Ϊ1-�������ʴ�Ϊ��1-������
��2��BΪCH3CH2CH2CHO�����еĹ�����Ϊȩ�����ʴ�Ϊ��ȩ����
��3�������Ϸ�����֪C+E��X�Ļ�ѧ��Ӧ������������ȡ������Ӧ��
�ʴ�Ϊ��������ȡ������
��4��AΪCH3CH2CH2CH2OH����Ӧ��ͬ���칹��C��CH3��3OH��CH3CH2CH��OH��CH3��CH3C��CH3��CH2OH�ȣ�
�ʴ�Ϊ��C��CH3��3OH��CH3CH2CH��OH��CH3��CH3C��CH3��CH2OH��������д��������
��5��XΪCH3CH2CH2COOCH2CH3����NaOH��Һ�ɷ���ˮ�⣬��Ӧ�ķ���ʽΪCH3CH2CH2COOCH2CH3+NaOH
| �� |
�ʴ�Ϊ��CH3CH2CH2COOCH2CH3+NaOH
| �� |
��6��DΪCH2=CH2���ɷ����Ӿ۷�Ӧ����Ӧ�ķ���ʽΪ
 ��
���ʴ�Ϊ��

���������⿼���л�����ƶϣ��Ǹ߿��еij������ͣ������е��Ѷȵ����⣮�����ۺ���ǿ�������߿�����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ��������������ּ������ѧ�����������ɡ��ܽ����������������������ѧ���������������ʹ���˼ά����������Ĺؼ��Ǹ���D�����Ʒ������ע����������Ϣ��

��ϰ��ϵ�д�
 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��
 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��

 X�Ļ�ѧ��Ӧ������
��Ӧ��
X�Ļ�ѧ��Ӧ������
��Ӧ��