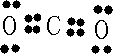��Ŀ����
A��B��C��D������������ˮ����ȫ���룬��������������±���
|
������ |
Na+��Al3+��Ba2+��H+��NH4+ |
|
������ |
SO42-��OH-��CO32-��Cl- |
�ֽ�������ʵ�飺
������A��Һ��B��Һ��Ϲ��ȿ����ɳ����ʹ̼�����ζ���壻
������A��Һ��C��Һ��Ͽ����ɳ����ң�
��A��Һ��B��Һ�����ܽ�����ң����������ܽ�����ס�
��ش�
��1��A�Ļ�ѧʽΪ_________������ʱ����pH��ȵ�A��Һ��D��Һ�ֱ�ϡ��10����pH�ֱ��Ϊa��b����a _______b(�>������=����<������
��2����������C��Һ�����գ�������ù���Ϊ_______ (�ѧʽ����
��3��C��Һ��D��Һ��Ӧ�����ӷ���ʽΪ_______
��4����B��Һ����μ��������������ʵ���Ũ�ȵ�NaOH��Һ���μӹ�����ˮ�ĵ���ƽ�⽫_______ (������������������ƶ�������������Һ�и�����Ũ���ɴ�С��˳��Ϊ____________________
��5����֪������Ksp=x����0.03mol��L-1��A��Һ��0.01mol��L-1��B��Һ�������ϣ������Һ��������ӵ�Ũ��Ϊ_______ (�ú�x�Ĵ���ʽ��ʾ����Ϻ���Һ����仯���Բ��ƣ���
��1��Ba(OH)2 <
��2��Al2O3
��3��2Al3++3CO32-+3H2O=2Al(OH)3��+3CO2��
��4������ c(Na+)= c(SO42-)> c(NH4+)> c(H+)> c(OH��)
��5��100 x L/mol
��������
�������������ʵ��ټ������ṩ�����ӣ��ɲ����̼�����ζ�����������ֻ��NH4+����A��B����һ�ֺ���NH4+����һ�ֺ���OH-������ʵ��ڣ���ȷ��A�к���OH-��B�к���NH4+������ʵ��ۣ������ҿ�����A��B�������ṩ�����ӣ��ó���ֻ����Al��OH��3��A�ɲ������ֳ�����AΪBa��OH��2����BΪ��NH4��2SO4���������ӹ��漰���ʵĿ����ԣ�CΪAlCl3����DΪNa2CO3��
��1��AΪǿ�BΪǿ�������Σ�ϡ����ͬ������A��pH�仯�����a<b��
��2����������AlCl3��Һʱ��Al3+ˮ��õ�Al(OH)3�������յõ�Al2O3��
��3��AlCl3��Na2CO3��ϣ���Ӧ��ʵ����Al3+��CO32-ˮ����ٽ�������ˮ�⡣
��4����NH4��2SO4��ǿ�������Σ���Һ�����ԣ��μ�NaOH��Һ�ٽ���NH4+��ˮ�⣬���ˮ�ĵ���ƽ�������ƶ������������NaOH��Һ��������ҺΪ�����ʵ�����Na2SO4����NH4��2SO4�Ļ����Һ��NH4+ˮ�⣬ʹ��Һ�����ԣ���˸�����Ũ���ɴ�С��˳��Ϊc(Na+)= c(SO42-)> c(NH4+)> c(H+)> c(OH��)��
��5��BaSO4��Ksp=x=c(Ba2+)��c(SO42-)��0.03mol��L-1��Ba(OH)2��Һ��0.01mol��L-1�ģ�NH4��2SO4��Һ�������ϣ� ����Һ���Ϊ1L����Ӧ����BaSO40.01mol��ʣ��Ba2+�����ʵ���Ϊ0.02mol����Һ��Ba2+��Ũ��Ϊ0.01mol��L-1����c(SO42-)= =
= =100 x L/mol��
=100 x L/mol��
���㣺����Ԫ�ػ�������ӹ��棬�ε�ˮ�⣬�����ܽ�ƽ�⣬���ӷ���ʽ��д�����ݡ�

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�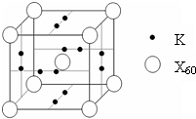 ��4��E����AB�γ���������E�ṩ
��4��E����AB�γ���������E�ṩ ��Bԭ��L��ĵ���������K���3����0.1mol C�����ܴ������û���2.24L��������״������ͬʱ���ĵ��Ӳ�ṹ�������ԭ�ӵĵ��Ӳ�ṹ��ͬ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
��Bԭ��L��ĵ���������K���3����0.1mol C�����ܴ������û���2.24L��������״������ͬʱ���ĵ��Ӳ�ṹ�������ԭ�ӵĵ��Ӳ�ṹ��ͬ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ�� +4H?��
+4H?��