��Ŀ����
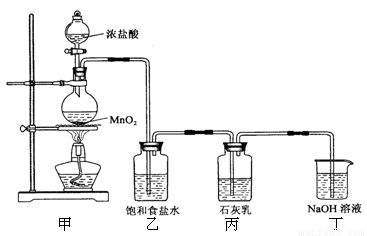
(12��)ij��ѧ��ȤС���ͬѧ����ͼ��ʾʵ��װ�ý���ʵ���о�(ͼ��a��b��c��ʾֹˮ��)������䷽���������ƻ�����;
(1)ʵ���ҽ�B��C��E�������� �� ����д���ƣ�Ϊԭ�Ͽ���ȡCl2��Ϊ�������о������Ļ�ѧ����������
(2) ����ʵ���ҳ��÷�����ȡ��������A��C��E�������ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ�飬ʵ����������������£�
| ʵ�� ��� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | Ʒ����Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ�� �ݲ��� | ������ˮ��Ӧ�IJ���������� |
ʵ����Ƴ���Ӧ�Ľ����Ƿ������ ________________������������˵�����ɣ���������������д���У���____________________________________________________.
(3)A��C��E�����������һ����ʵ�飬����֤Cl����Br���Ļ�ԭ��ǿ�����йط�Ӧ�����ӷ���ʽΪ��____________________________________��
(4)B��D��Eװ����������B��ʢװŨ�����ͭƬ(�����п����ϰ���)�����Ƶò�����NO2�й�ʵ�飮
��B�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________
������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ��________���ٴ�ֹˮ��________��ʹ�ձ��е�ˮ�����Թܶ��Ŀ��ܵIJ����� ��
(12��) 26.(1)������ơ�Ũ���ᣨ�����������Ĵ𰸣���2�֣�(2) ��������û������֤�������������Ư���ԡ�
����������ȡ�������к���HCl���壬HCl����ˮ������̼�����Ʒ�ĩ��Ӧ�������ݣ�4�֣�
(3)MnO2 +4H+ +2Cl-  Mn2+ + 2H2O +Cl2��, Cl2 + 2Br-��2Cl- + Br2��2�֣�
Mn2+ + 2H2O +Cl2��, Cl2 + 2Br-��2Cl- + Br2��2�֣�
(4)��Cu��4HNO3(Ũ) �� Cu(NO3)2��2NO2����2H2O��2�֣�
��a��b����c��˫�ֽ���(����)�Թܶ���ʹ�Թ��������ݳ���NO2��ˮ�Ӵ��������ձ��е�ˮ�������Թܶ��У�2�֣��������������Ĵ𰸣�
����

 �����Ļ�������ҵϵ�д�
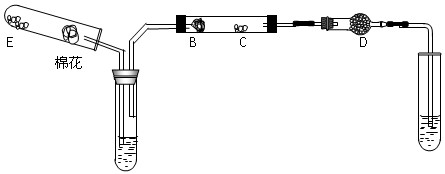
�����Ļ�������ҵϵ�д�����С�12�� ��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���

�밴Ҫ����գ�
��1������Bװ�ÿ���ȡ��������______________________��д�����ּ��ɣ���
��2��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮
���ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ
�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ���������� |

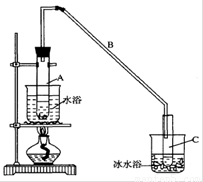
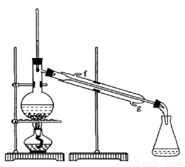
 5Cl��+ClO3��+3H2O������ȤС�����������ʵ��װ�ã�����ʵ�顣
5Cl��+ClO3��+3H2O������ȤС�����������ʵ��װ�ã�����ʵ�顣