��Ŀ����
20��ijͬѧ��NaCl��������200mL 1mol/L��NaCl��Һ����ش��������⣮��1����������ƽ��ȡNaCl���壬��������11.7 g��
��2��ʵ���������ȷ˳���Ǣܢޢۢݢ٢ڣ�����ţ���
�ټ���������ˮ��Һ���̶���1��2 cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����̶������У�
�ڸǺ�����ƿƿ�������ҡ�ȣ�
�۰�������Һ��ȴ�����º�С��ת������ƿ�У�
�ܳ������壻
������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ��
�ްѳ����õĹ������С�ձ��У�����������ˮ�ܽ⣮
��3�����в�������ҺŨ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
a��������Һʱ������ƿδ�����Ӱ��
b�����ƹ����У�û��ϴ���ձ��Ͳ�������ƫ��
c������ʱ�����ӿ̶��ߣ�ƫ��
d�������ƺõ���Һ��ȡ��10mL����Ӱ�죮
���� ��1����������m=CVM�����㣻
��2���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��3������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1������ʵ������200mL������ƿ����Ӧѡ��250mL������ƿ�������Ƴ�����250mL��1mol/l����Һ��������Ȼ�����Һ������m=CVM=1mol/L��0.25L��58.5g/mol=11.7g���ʴ�Ϊ��11.7g��
��2���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������֪��ȷ�IJ���˳���ǣ��ܢޢۢݢ٢ڣ��ʴ�Ϊ���ܢޢۢݢ٢ڣ�
��3��a��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죻
b�����ƹ����У�û��ϴ���ձ��Ͳ��������ᵼ�����ʵ���ʧ����������Һ��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
c������ʱ�����ӿ̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
d��������Һ�Ǿ�һ�ȶ��ģ���ȡ��10mL�����ҺŨ����Ӱ�죬�ʴ�Ϊ����Ӱ�죮
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���1�������淽���У�A��ʾ�ɷ�Һ©������ƿ��ɵ����巢���������ڴ���ϵ�A����ɸ÷�Ӧ��ʵ��װ��ʾ��ͼ���г�װ�á����ӽ��ܲ��ػ�����β���������ֱ��뻭������Ҫ���ȵ������·��á��������������������ÿ�������·������ĸB��C����������ѡ�õ��������������ޣ����ױ�ʾ���£�

��2�����ݷ����е�װ��ͼ�ף��ڴ������д�±����ɲ�������
| ������� | �������������� | ���� |
| A | �������ơ�Ũ���� | ����SO2 |
��SO2�����Ը��������Һ������Ӧ�����ӷ���ʽΪ��5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
�ڷ�Ӧ������Һ�Ϻ�ɫ��ʧ����û�м�ʱֹͣͨ�������õ�SO2����ƫ�ͣ�ѡ���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�������Ը��������Һ���ΪV1mL��Ũ��Ϊcmol/L�������������ӵ�ˮ�����ΪV2mL��������ɱ�״���µ����������c��V1��V2��ʾSO2������ٷֺ���Ϊ=$\frac{56{V}_{1}c}{56{V}_{1}c+V{\;}_{2}}$��100%��
| A�� | �Է�Ӧ��ϵ���� | B�� | ���������ķ�Ӧ���� | ||
| C�� | ������������ͭ��Һ | D�� | �������۴�����Ƭ |
| A�� | ��������Һ | B�� | ����������Һ | C�� | ����������Һ | D�� | ������ |
| A�� | ���õķ�ɢϵ��������Һ | |
| B�� | ���õķ�ɢϵ�з�ɢ��ΪFe2O3 | |
| C�� | �÷�ɢϵ�ܲ��������ЧӦ�����н���������� | |
| D�� | ���÷�ɢϵΪ���壬�ҽ���ֱ����Ϊ������������ֱ�� |

 ��
��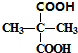 +2C2H5OH$��_{��}^{Ũ����}$
+2C2H5OH$��_{��}^{Ũ����}$ +2H2O��
+2H2O�� ������NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ
������NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
�� �ס��ҡ��������ֳ������ʣ�A��B��C�dz��������B�ڳ�����ΪҺ̬����һ����������������֮��Ĺ�ϵ��ͼ��
�ס��ҡ��������ֳ������ʣ�A��B��C�dz��������B�ڳ�����ΪҺ̬����һ����������������֮��Ĺ�ϵ��ͼ�� ����-��ԭ��Ӧ����ѧ��ѧ��һ����Ҫ�ķ�Ӧ���ͣ����ճ������кܳ�������ش𣺣�1���ڷ�Ӧ3Cu+8HNO3��ϡ���T3Cu��NO3��2+2NO��+4H2O�У������������4.48L����ʱ�����ĵ���ͭ19.2g����ʱת����0.6mol���ӣ�
����-��ԭ��Ӧ����ѧ��ѧ��һ����Ҫ�ķ�Ӧ���ͣ����ճ������кܳ�������ش𣺣�1���ڷ�Ӧ3Cu+8HNO3��ϡ���T3Cu��NO3��2+2NO��+4H2O�У������������4.48L����ʱ�����ĵ���ͭ19.2g����ʱת����0.6mol���ӣ�