��Ŀ����
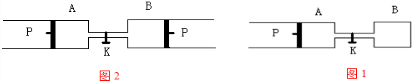
 ��ͼ��ʾ��PΪһ�����ɻ����Ļ������ر�K���ֱ�������A��B�о�������2mol X��2mol Y����ʼʱ��VA=a L��VB=0.8a L����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥʹ������ڵ������£��������и��Է���������Ӧ��3X��g��+3Y��g��?2Z��g��+2W��g�����ﵽƽ��ʱ��VB=0.6a L��
��ͼ��ʾ��PΪһ�����ɻ����Ļ������ر�K���ֱ�������A��B�о�������2mol X��2mol Y����ʼʱ��VA=a L��VB=0.8a L����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥʹ������ڵ������£��������и��Է���������Ӧ��3X��g��+3Y��g��?2Z��g��+2W��g�����ﵽƽ��ʱ��VB=0.6a L���Իش��������⣺
��1��B��X��ת����Ϊ
75%
75%
����2��A��B�е�X��ת���ʵĹ�ϵ��A
��
��
B�����������=������������������������������ͬ��B�������A��С����B�������ѹǿ��A�еĴ�������Ӧ����ѹƽ�����ƣ�B��������ת���ʴ�
����������ͬ��B�������A��С����B�������ѹǿ��A�еĴ�������Ӧ����ѹƽ�����ƣ�B��������ת���ʴ�
����3��ƽ��ʱA��B�л�������ƽ����Է��������Ĺ�ϵ�ǣ�MA
��
��
MB�����������=��������������4�����Ҫ�����ƽ��ʱB�л��������ܶȣ������ٻ���Ҫ֪����������
AB
AB
����д��ĸ����MX��MY��MZ��MW�ֱ��ʾX��Y��Z��W��Ħ������������MX ��MY ��MZ ��MW
A���ۺ͢ܣ�B���ٺ͢ڣ�C���ٺۣ͢�D���ڢۢܣ�
��5����K��һ��ʱ���Ӧ�ٴδﵽƽ�⣬��B�����Ϊ
0.2a
0.2a
L����������1��BΪ���º�ѹ���������֮�ȵ������ʵ���֮�ȣ����ƽ��ʱB�л����������ʵ�������ת����X�����ʵ���Ϊ��xmol����������ʽ�з��������������ת���ʵĶ������x��ת���ʣ�
��2��A�ݻ����䣬��B�ݻ���С��A������ѹǿС��B��ѹǿ��ƽ�����淴Ӧ�����ƶ���
��3��AΪ���º���������BΪ���º�ѹ������B���������С��ѹǿ����ƽ��������У��������ʵ�����С����������Ħ��������������жϣ�
��4������Ħ������������������������ܶ����������������������������������Ҫ֪�����ڵ�Ħ��������
��5����K�ﵽƽ��ʱ��ƽ����ϵ�������ԭB��������ͬ�����ʵ�ת������ͬ��ƽ��ʱ����ֵĺ�����ͬ��
��2��A�ݻ����䣬��B�ݻ���С��A������ѹǿС��B��ѹǿ��ƽ�����淴Ӧ�����ƶ���
��3��AΪ���º���������BΪ���º�ѹ������B���������С��ѹǿ����ƽ��������У��������ʵ�����С����������Ħ��������������жϣ�
��4������Ħ������������������������ܶ����������������������������������Ҫ֪�����ڵ�Ħ��������
��5����K�ﵽƽ��ʱ��ƽ����ϵ�������ԭB��������ͬ�����ʵ�ת������ͬ��ƽ��ʱ����ֵĺ�����ͬ��
����⣺��1��BΪ���º�ѹ����Ӧǰ�����֮�ȵ������ʵ���֮�ȣ���ƽ���B�л����������ʵ���Ϊn����
0.8aL��0.6aL=4mol��n�����n=3mol��
3X��g��+3Y��g��?2Z��g��+2W��g����
��ʼ��2mol 2mol 0mol 0mol
ת����xmol xmol
xmol
xmol
ƽ�⣺��2-x��mol ��2-x��mol
xmol
xmol
���ԣ���2-x��mol+��2-x��mol+
xmol+
xmol=3mol��
���x=1.5��
N2��ת����Ϊ
��100%=75%��
�ʴ�Ϊ��75%��
��2��A�ݻ����䣬��B�ݻ���С������A������ѹǿС��B��ѹǿ��ƽ�����淴Ӧ�����ƶ���X2��ת����С��B��X2��ת���ʣ�
�ʴ�Ϊ��С�ڣ�����������ͬ��B�������A��С����B�������ѹǿ��A�еĴ�������Ӧ����ѹƽ�����ƣ�B��������ת���ʴ�
��3��AΪ���º���������BΪ���º�ѹ��������Ӧ�����������������䣬B���������С��ѹǿ����ƽ��������У��������ʵ�����С��B�л�������ƽ����Է���������ƽ��ʱA��B�л�������ƽ����Է��������Ĺ�ϵ��MA��MB��
�ʴ�Ϊ������
��4�����Ҫ�����ƽ��ʱB�л��������ܶȣ������ٻ���Ҫ֪���������ǻ��������������ԣ���Ӧ����������Ħ�������ǽ������ݣ��٢ڻ�ۢܣ�
�ʴ�Ϊ��AB��
��5����K�ﵽ��ƽ�⣬�ɵ�ЧΪ��ʼ��K�ﵽ��ƽ�⣬��ԭB��ƽ���Ч��������ת������ͬ���������ʵ�����Ϊ2mol��NH3�����ʵ���ΪԭB��2����ƽ����ϵ�������ԭB��������ͬ��ƽ��ʱ����ֵĺ������䣬��ʱ�����ڻ����������ʵ���ΪԭB��2�������֮�ȵ������ʵ���֮�ȣ������������ΪԭBƽ��ʱ�����2������1.2aL���ʴ�ʱB�����1.2aL-aL=0.2aL��
�ʴ�Ϊ��0.2a��
0.8aL��0.6aL=4mol��n�����n=3mol��
3X��g��+3Y��g��?2Z��g��+2W��g����
��ʼ��2mol 2mol 0mol 0mol
ת����xmol xmol
| 2 |
| 3 |
| 2 |
| 3 |
ƽ�⣺��2-x��mol ��2-x��mol
| 2 |
| 3 |
| 2 |
| 3 |
���ԣ���2-x��mol+��2-x��mol+
| 2 |
| 3 |
| 2 |
| 3 |
���x=1.5��
N2��ת����Ϊ
| 1.5mol |
| 2mol |
�ʴ�Ϊ��75%��
��2��A�ݻ����䣬��B�ݻ���С������A������ѹǿС��B��ѹǿ��ƽ�����淴Ӧ�����ƶ���X2��ת����С��B��X2��ת���ʣ�
�ʴ�Ϊ��С�ڣ�����������ͬ��B�������A��С����B�������ѹǿ��A�еĴ�������Ӧ����ѹƽ�����ƣ�B��������ת���ʴ�
��3��AΪ���º���������BΪ���º�ѹ��������Ӧ�����������������䣬B���������С��ѹǿ����ƽ��������У��������ʵ�����С��B�л�������ƽ����Է���������ƽ��ʱA��B�л�������ƽ����Է��������Ĺ�ϵ��MA��MB��
�ʴ�Ϊ������
��4�����Ҫ�����ƽ��ʱB�л��������ܶȣ������ٻ���Ҫ֪���������ǻ��������������ԣ���Ӧ����������Ħ�������ǽ������ݣ��٢ڻ�ۢܣ�
�ʴ�Ϊ��AB��
��5����K�ﵽ��ƽ�⣬�ɵ�ЧΪ��ʼ��K�ﵽ��ƽ�⣬��ԭB��ƽ���Ч��������ת������ͬ���������ʵ�����Ϊ2mol��NH3�����ʵ���ΪԭB��2����ƽ����ϵ�������ԭB��������ͬ��ƽ��ʱ����ֵĺ������䣬��ʱ�����ڻ����������ʵ���ΪԭB��2�������֮�ȵ������ʵ���֮�ȣ������������ΪԭBƽ��ʱ�����2������1.2aL���ʴ�ʱB�����1.2aL-aL=0.2aL��
�ʴ�Ϊ��0.2a��
������������Ҫ���黯ѧƽ����㣬�ѶȽϴ�ע���Чƽ��������ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ