��Ŀ����
��һ�����İ�������粒�������ij�ݻ��㶨����������У�����
��Ӧ��H2NCOONH4(s) 2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��
2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��
A.��T2��T1����÷�Ӧ�Ħ�H��0
B�����������N2��H2NCOONH4��������
C��NH3�����������ʱ��˵���÷�Ӧ�ﵽƽ��
D��T1��T2ʱ��ת����H2NCOONH4�����ʵ�����n(T2)��2��n(T1)
��Ӧ��H2NCOONH4(s)
 2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��
2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��| �¶� | ƽ��Ũ��(mol��L��1) | |
| | c(NH3) | c(CO2) |
| T1 | 0.1 | |
| T2 | | 0.1 |
A.��T2��T1����÷�Ӧ�Ħ�H��0
B�����������N2��H2NCOONH4��������
C��NH3�����������ʱ��˵���÷�Ӧ�ﵽƽ��
D��T1��T2ʱ��ת����H2NCOONH4�����ʵ�����n(T2)��2��n(T1)
D
���۷�Ӧ�Ƿ�ﵽƽ��״̬��NH3�������������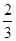 ����C����T2ʱc(NH3)��c(CO2)����T1ʱ��2�����������ݻ��ֺ㶨���䣬����T2ʱת����H2NCOONH4��T1ʱ��2����D��ȷ��
����C����T2ʱc(NH3)��c(CO2)����T1ʱ��2�����������ݻ��ֺ㶨���䣬����T2ʱת����H2NCOONH4��T1ʱ��2����D��ȷ��
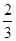 ����C����T2ʱc(NH3)��c(CO2)����T1ʱ��2�����������ݻ��ֺ㶨���䣬����T2ʱת����H2NCOONH4��T1ʱ��2����D��ȷ��
����C����T2ʱc(NH3)��c(CO2)����T1ʱ��2�����������ݻ��ֺ㶨���䣬����T2ʱת����H2NCOONH4��T1ʱ��2����D��ȷ��
��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�
�����Ŀ
 2NH3(g) ��H����92.4 kJ/mol���ݴ˻ش��������⣺
2NH3(g) ��H����92.4 kJ/mol���ݴ˻ش��������⣺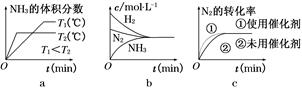
 CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��
CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��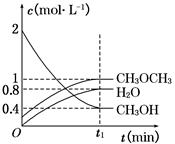
 2XY3(g) ��H=��92.6 kJ��mol-1��ʵ�����й��������±���
2XY3(g) ��H=��92.6 kJ��mol-1��ʵ�����й��������±���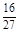
 TaI4(g)��S2(g)����H��0��t minʱ����0.1 mol TaI4������˵����ȷ����
TaI4(g)��S2(g)����H��0��t minʱ����0.1 mol TaI4������˵����ȷ����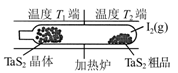
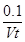 mol��L��1��min��1
mol��L��1��min��1 C(g)��D(g)��5 min���ƽ�⣬��֪�����ʵ�ƽ��Ũ�ȵĹ�ϵΪca(A)��c(B)��c(C)��c(D)�������¶Ȳ��������£����������������Ϊԭ����10����A��ת����û�з����仯����B��ת����Ϊ�� ��
C(g)��D(g)��5 min���ƽ�⣬��֪�����ʵ�ƽ��Ũ�ȵĹ�ϵΪca(A)��c(B)��c(C)��c(D)�������¶Ȳ��������£����������������Ϊԭ����10����A��ת����û�з����仯����B��ת����Ϊ�� �� C(g)��D(g)�ﵽƽ��������¶�����
C(g)��D(g)�ﵽƽ��������¶����� 2HI(g)�Ѵ�ƽ��״̬��������������
2HI(g)�Ѵ�ƽ��״̬��������������  2HI(g)����H<0�����ﵽƽ���t1ʱ�̸ı䷴Ӧ��ijһ����(������������ʵ�������)�����������ѹǿ����������˵������ȷ���� (����)
2HI(g)����H<0�����ﵽƽ���t1ʱ�̸ı䷴Ӧ��ijһ����(������������ʵ�������)�����������ѹǿ����������˵������ȷ���� (����)